- Home
- News
- Spotlight on Science
- Surface distortion...
Surface distortion descriptor relates catalytic activity to the proportion of surface defects in oxygen reduction reaction electrocatalysis
30-08-2018
High-energy X-rays have revealed the fundamental role played by surface defects in state-of-the-art platinum-nickel nanocatalysts for the oxygen reduction reaction and unveiled a new descriptor that can be used to design efficient and durable universal electrocatalysts.
The oxygen reduction reaction (ORR) is a key reaction in many important chemical processes such as corrosion and it is widely employed in sensor technologies and energy conversion and storage systems. Surprisingly, recent results on ‘structurally-disordered’ catalysts, such as hollow PtNi nanoparticles, dealloyed porous PtNi nanoparticles or PtNi aerogels, have shown desirable enhancement of the ORR activity (10-12-fold enhancement over pure Pt/C) [1-2]. To date, the mechanisms of this unexpected ORR activity enhancement remain unclear, and prevent further development of this vital technology for a carbon-free energy future.
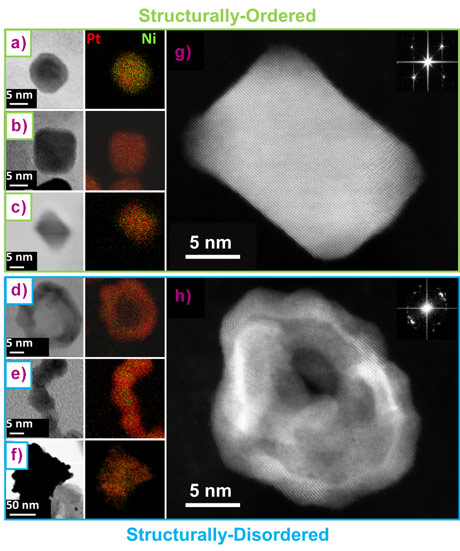
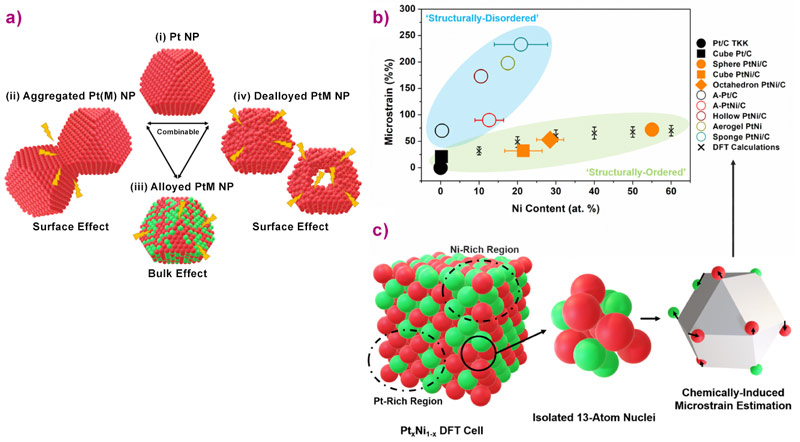
To get fundamental understanding of this unexpected enhancement, different state-of-the-art PtNi/C nanocatalysts were synthesised with distinct atomic composition, size, shape and density of surface defects but all featuring highly desirable ORR activity. As shown by Figure 1, the catalysts can be separated into two categories: the structurally-ordered, composed of monocrystalline nanoparticles of different chemical compositions and shapes (spherical, cubic and octahedral), and the structurally-disordered, composed of multi-grained nanoparticles featuring a high proportion of grain boundaries. Their structural disorder was quantified experimentally at beamline ID31, using the values of microstrain (a parameter accessible by the Rietveld refinement of wide-angle X-ray scattering (WAXS) pattern) that is representative of the local distortion of a crystal lattice. As shown in Figure 2a, grain boundaries, inhomogeneous alloying, and electrochemical surface leaching (also referred as dealloying) cause microstrain. Interestingly, microstrain was also detected in all electrocatalysts (including the supposed structurally-ordered ones), except for pure monocrystalline Pt nanoparticles (Figure 2b). Because microstrain is a bulk measurement of structural disorder, and only a relative measurement, the bulk and surface components must be disentangled as only the surface of the catalyst is involved in heterogeneous catalysis.
Density functional theory (DFT) calculations were used to estimate the bulk contribution of microstrain, which is caused by the inhomogeneous distribution of the alloying element within the Pt lattice (chemical disorder). As shown by Figure 2c, this contribution only depends on the Ni content, and the calculated values match those measured for structurally-ordered catalysts (Figure 2b), suggesting that the chemical disorder is the only source of microstrain for this class of materials. This chemical disorder was subtracted from the overall disorder to get the surface distortion (SD) descriptor (see associated publication for further details), which gives a semi-quantification of a nanocatalyst surface defectiveness.
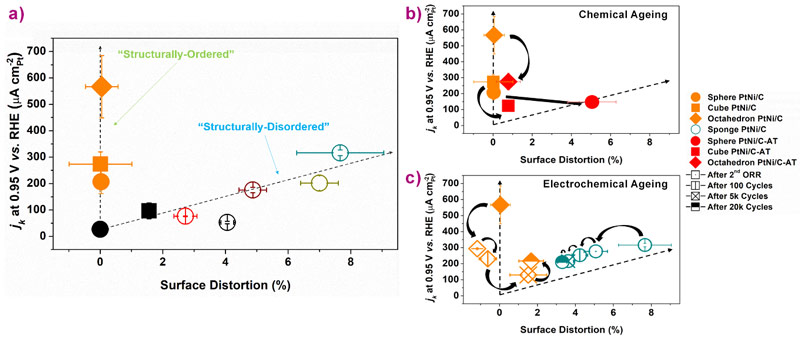
This new SD descriptor was used to rationalise the ORR activity enhancement of the two classes of materials (structurally-ordered and structurally-disordered). Figure 3a shows that the ORR activity of the fresh nanocatalysts features two branches when plotted as a function of the SD descriptor value. On the left branch, (where SD is almost null) various ORR kinetics can be reached, whereas on the right branch, the ORR kinetics increases almost linearly with SD. Null SD is evidence of homogeneous, perfectly ordered surfaces initially tailored to weakly chemisorb ORR intermediates and enhance the catalytic activity. The chemisorption energy of ORR intermediates controls the ORR rate according to the Sabatier principle [3].
Increasing values of SD point towards buckled surfaces and local variations of the coordination number [4]. For these nanocatalysts, each catalytic site has a unique configuration and features its own ORR activity. The overall increase in activity of these distorted surfaces is controlled by the proportion of sites that randomly features the optimal affinity with oxygenated species. Therefore, the SD descriptor can be used for both structurally-ordered and structurally-disordered materials, greatly increasing the predictive power of this approach.
Beyond its unifying character, the SD descriptor was also used to shed light on the long-term stability of the two different approaches. Figure 3b-c provides clear-cut evidence that the SD descriptor is robust enough to capture the surface state upon (electro)chemical ageing. The results suggest that surface distortion unavoidably occurs in simulated proton-exchange membrane fuel cell (PEMFC) cathode environments and controls the ORR activity on the long-term. Consequently, and contrarily to the traditional approach, a strategy that consists of enhancing initial structural disorder opens a new route toward more efficient and durable ORR electrocatalysts and will accelerate the development of PEMFC devices.
Principal publication and authors
Surface distortion as a unifying concept and descriptor in oxygen reduction reaction electrocatalysis, R. Chattot (a,b), O. Le Bacq (c), V. Beermann (d), S. Kühl (d), J. Herranz (e), S. Henning (e), L. Kühn (f), T. Asset (a), L. Guétaz (g), G. Renou (c), J. Drnec (b), P. Bordet (i), A. Pasturel (c), A. Eychmüller (f), T.J. Schmidt (e,j), P. Strasser (d), L. Dubau (a) and F. Maillard (a), Nature Materials, (2018); doi: 10.1038/s41563-018-0133-2.
(a) Univ. Grenoble Alpes, CNRS, Grenoble INP, Univ. Savoie Mont Blanc, LEPMI, Grenoble (France)
(b) ESRF
(d) Electrochemical Energy, Catalysis and Material Science Laboratory, Department of Chemistry, Technische Universität Berlin (Germany)
(e) Electrochemistry Laboratory, Paul Scherrer Institut, Villigen (Switzerland)
(f) Physical Chemistry, Technische Universität Dresden (Germany
(g) Univ. Grenoble Alpes, CEA, Liten, Grenoble (France)
(i) CNRS, Institut Néel, Grenoble (France)
(j) Laboratory of Physical Chemistry, ETH Zurich (Switzerland)
References
[1] L. Dubau et al., ACS Catal. 6, 4673–4684 (2016).
[2] R. Chattot et al., ACS Catal. 7, 398–408 (2017).
[3] J.K. Nørskov et al., J. Phys. Chem. B 108, 17886–17892 (2004).
[4] F. Calle-Vallejo et al., Science 350, 185–190 (2015).