- Home
- Users & Science
- Find a beamline
- ID22 - High resolution powder diffraction beamline
- Science at ID22
- Examples: Structural Studies
Examples: Structural Studies
Structural studies
Here you will find a cross-section of the studies carried out on the high-resolution powder diffraction beamline. Experiments were carried out on ID22 (since 2014), and ID31 (2002 - 2013). Examples come from:
- Inorganic and organic chemistry:
-
Framework structures:
- Zeolite TNU-9,
- Aluminosilicate ECS-14,
- Metal-organic frameworks MIL-100 and MIL-146(Li)
-
Proteins:
- Posin,
- Human insulin,
- Bovine insuline,
- Urate oxidase
- Read more on methods for structural biology
Inorganic and organic chemistry
Ba3NaRu2O9
There have been many crystallographic studies using ID31. An example of note is the recent study of Ba3NaRu2O9 as a function of temperature [1]. This material has a quasimolecular structure with Ru5.5+2O9 dimers in a hexagonal perovskite structure with hexagonal (P63/mmc) symmetry at room temperature in which all Ru sites are equivalent. Magnetic susceptibility measurements show a first-order transition at 210 K to a reportedly C-centred orthorhombic structure consistent with the opening of a charge or spin gap. ID31 measurements however resolved additional splitting of peaks (figure 1) showing the true structure to be monoclinic, with the appearence of weak reflections indicating the space group to be P2/c. There are two symmetry inequivalent charge-ordered Ru5+2O9 and Ru6+2O9 dimers, (the oxidation states assessed from Ru – Ru distances, the magnetic behaviour, and theoretical modelling). Below 40 K, lattice parameters derived from ID31 and neutron diffraction differ significantly, and it transpires that the high X-ray flux causes a further electronic transition to a charge-disordered state with only one Ru site (space group C2/c). At 10 K the transformation is quite rapid, with an exponential time constant of 78 s, which could be followed by rapid scans on ID31. The energy balance between the “charge-melted” and charge-ordered states is evidently quite delicate; the P2/c ordered state is recovered on warming to above 40 K, including recovery of the microstrain.
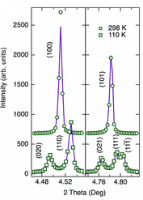
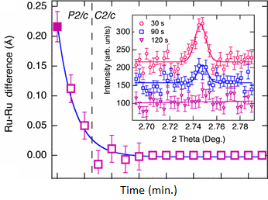
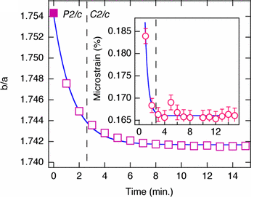
Figure 1. (left), splitting and peak broadening in Ba3NaRu2O9 on cooling to the charge-ordered monoclinic phase; (middle) difference between the Ru-Ru distance in the two dimers as a function of irradiation time at 10 K; the inset shows the disappearance of the h+k = odd superstructure peaks; (right) decrease in the b/a ratio and microstrain (inset) determined from the tanq contribution to the Lorenzian peak shape, [1].
[1] Charge order at the frontier between the molecular and solid states in Ba3NaRu2O9. Kimber S.A.J., Senn M.S., Fratini S., Wu H., Hill A.H., Manuel P., Attfield J.P., Argyriou D.N., Henry P.F. Phys. Rev. Lett. 108, 217205, (2012).
(top)
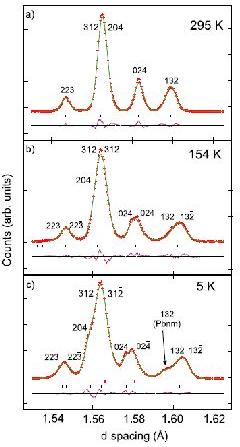 Perovskite oxide SmVO3
Perovskite oxide SmVO3
Another example of note is the study of the perovskite oxide SmVO3 [2] as a function of temperature. The very high resolution (at short wavelength) allows subtle changes in the diffraction profile to be observed, figure 2, and the coexistence of two phases at low temperature to be ascertained (one orthorhombic, the other monoclinic, angle = 90.019(4)°), representing different electronic orbital ordering schemes, as supported by the refined V–O bond lengths. The phase coexistence is triggered by an antiferromagnetic ordering of the vanadium spins near 130 K, below an initial orbital ordering near 200 K. The phase coexistence is the result of the intermediate ionic size of samarium coupled to exchange striction at the vanadium spin ordering.
Figure 2. Successive phases of SmVO3 with (a) T > TOO: Pbnm (Rwp = 0.078), (b) TN < T < TOO: P21/b11, (c) T < TN: Pbnm (upper markers) and P21/b11 (lower markers (Rwp = 0.0517).
[2] Evidence for Electronic Phase Separation between Orbital Orderings in SmVO3. M. H. Sage, G. R. Blake, G. J. Nieuwenhuys, and T. T. M. Palstra, Phys. Rev. Lett., 96, 036401 (2006).
(top)
Cystal structure of the 9-ethylbicyclo[3.3.1]nona-9-ol
The crystal structure of the organic molecule 9-ethylbicyclo[3.3.1]nona-9-ol was solved using direct methods from ID31 data [3]. The diffraction pattern measured originally on BM16 could be indexed and the space group determined (Pbca), but with four independent molecules in the unit cell (48 non-hydrogen atoms) and a cell volume near 8000 Å3, no solution for the structure could be found, despite excellent looking powder data extending below 1 Å in d spacing. On ID31 data measured at five temperatures were combined to define a set of intensities from which the structure could be solved by direct methods, exploiting the anisotropic thermal expansion of the unit cell. In this approach, the anisotropic lattice changes modify the degree of overlap between adjacent reflections. By fitting a single set of intensities to the multiple data sets, the number and the reliability of the individual peak intensities that can be extracted from the powder profiles are increased. For such an approach, high-resolution data are essential as the shifts in relative peak positions with temperature can be rather small and do not affect peak overlap significantly unless the peaks are inherently narrow. Anisotropic lattice changes have also been exploited in powder diffraction studies on proteins, either by precipitating the protein at different pH values, and/or by exploiting radiation damage.
[3] Solving Larger Molecular Crystal Structures from Powder Diffraction Data by Exploiting Anisotropic Thermal Expansion. M. Brunelli, J. P. Wright, G. B. M. Vaughan, A. J. Mora, and A. N. Fitch, Angew. Chem. Int. Ed., 42, 2029 (2003).
(top)
Framework structures
Complex zeolite TNU-9
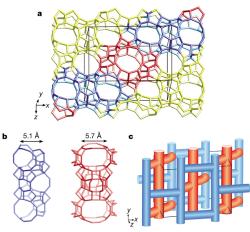
Crystal structures can also be solved from powders by incorporating known structural-chemical information into the procedure. Thus molecular structures can be solved by using a global minimisation procedure (e.g. simulated annealing) to position molecules or fragments in the unit cell, varying position, orientation and torsion angles, as appropriate. Such methods are now quite standard for powder crystallographers, and laboratory data is often adequate, provided the angular resolution is good enough to determine the unit cell and space group accurately. Chemical information about zeolite-framework co-ordination was also used in the FOCUS method to solve the structure of the complex zeolite TNU-9, figure 4, [4] from ID31 data, in conjunction with electron diffraction for indexing and space group determination, (C2/m, a = 28.2219 Å, b = 20.0123 Å, c = 19.4926 Å, β = 92.33°) and very high quality HRTEM images that yielded starting phases for 258 reflections. The structure has a total of 76 atoms (of which 24 are framework Si).
Figure 4. The structure of TNU-9 [3].
[4] Complex zeolite structure solved by combining powder diffraction and electron microscopy. F. Gramm, C. Baerlocher, L. B. McCusker, S. J. Warrender, P. A. Wright, B. Han, S. B. Hong, Z. Liu, T. Ohsuna, and O. Terasaki, Nature, 444, 79–81 (2006).
(top)
Crystal structure of ECS-14, a crystalline microporous hybrid organic-inorganic aluminosilicate
The crystal structure of ECS-14, a crystalline microporous hybrid organic-inorganic aluminosilicate exploiting 1,4-bis-(triethoxysilyl)-benzene as the source of silica - composition determined analytically as Na0.80B0.04Si1.22Al1.00(C6H4)0.59O3.80·2H2O - was solved [5] from measurements made at 25 keV (0.496 Å wavelength), via direct methods and the EXPO2009 package. The unit cell is hexagonal, space group P6/mcc with a unit cell a = 14.213 Å, c = 27.208 Å , V = 4760 Å3, Z = 24. The structure contains a system of linear channels with 12-membered ring openings resembling the pore architecture of the AFI zeolite framework type. Anisotropic broadening of the peaks arising from the plate-like morphology of the crystallites needed to be modelled in fitting the experimental pattern. Extraframework sodium ions and water molecules could be located in the Fourier maps during the Rietveld refinement.
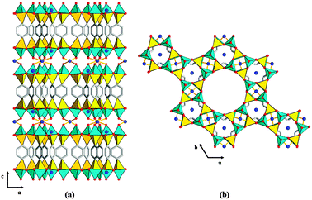
Figure 5. (left) Rietveld refinement of ECS-14; (right) projections of the structure down (a) [010] and (b) down [001], [5].
[5] A highly crystalline microporous hybrid organic-inorganic aluminosilicate resembling the AFI-type zeolite. Bellussi G., Millini R., Montanari E., Carati A., Rizzo C., Parker Jr W.O., Cruciani G., De Angelis A., Bonoldi L., Zanardi S. Chem. Comm. 48, 7356, (2012).
(top)
Enormous metal-organic-framework material MIL-100
Another example is the solution of the enormous metal-organic-framework material MIL-100, with a cubic unit cell of length 72.906 Å and a unit cell volume of 387,516 Å3 [6a], via a computational scheme for predicting how the structure could be built up by the chemical combination of smaller subunits. Comparison of the calculated intensities with those observed on ID31 showed which of the predicted structures was correct. The even bigger (a = 88.869 Å, V = 701860.3 Å3) MIL-101 [6b] was solved following a similar procedure.
[6a] A Hybrid Solid with Giant Pores Prepared by a Combination of Targeted Chemistry, Simulation, and Powder Diffraction. G. Férey, C. Serre, C. Mellot-Draznieks, F. Millange, S. Surblé, J. Dutour, and I. Margiolaki, Angew. Chem. Int. Ed., 43, 6296 (2004).
[6b] A Chromium Terephthalate-Based Solid with Unusually Large Pore Volumes and Surface Area. G. Férey, C. Mellot-Draznieks, C. Serre, F. Millange, J. Dutour, S. Surblé, and I. Margiolaki, Science, 309, 2040 (2005).
(top)
Lithium-organic framework material MIL-146(Li)
The triclinic structure of the lithium-organic framework material MIL-146(Li) and its hydrated analogue were solved from ID31 data by indexing the powder patterns and then by simulated annealing, treating the tetracarboxylate linking ion as a rigid body. The structures were completed by Rietveld refinement and the calculation of Fourier maps [7]. MIL-146 is obtained irreversibly by heating the structurally distinct material MIL-145. Such materials are of particular interest because of their chemical novelty, and also because the metal centre is lithium, so a light non-transition element, offering perhaps the possibility of high gravimetric capacity for the adsorption of gases. Dehydrated MIL-146 contains four- and three-coordinate lithium-ion centres with the coordinatively unsaturated trigonal planar lithium able to bind water reversibly with its crystallinity maintained. Moreover, this phase shows a marked preference for adsorption of CO2 over N2.
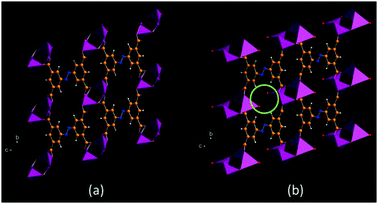
Figure 7. (a) Dehydrated and (b) hydrated MIL-146. Coordinated water molecules are indicated in (b), [7].
[7] A lithium-organic framework with coordinatively unsaturated metal sites that reversibly binds water. El Osta R., Frigoli M., Marrot J., Guillou N., Chevreau H., Walton R.I., Millange F. Chemical Communications 48, 10639,(2012).
(top)
Proteins
SH3 domain of ponsin
Recently it has become apparent that the high resolution powder diffraction data can be used to refine the structures of small proteins (like lysozyme) by the Rietveld method when combined with extensive stereochemical restraints [8a]. The in-house team of I. Margiolaki (now University of Patras, Greece) and J. Wright (now at ID11, ESRF) has pushed powder protein diffraction to new frontiers, using ID31 data. Recently (in collaboration with N. Pinotsis and Matthias Wilmanns at EMBL Hamburg) the previously unknown structure of the SH3 domain of ponsin (comprising 67 amino acids, space group P212121, V = 64879 Å3) was solved by molecular replacement from a model protein molecule with only a 40% correspondence between the amino-acid residues [8b]. Intensities were extracted by fitting to four data sets exploiting anisotropic differences in unit cell parameters induced by precipitating two batches of sample, and by radiation damage. Reflections with a reasonable signal-to-noise ratio were extracted up to a d spacing resolution of about 2.4 Å. In the final stages of the reconstruction of the electron density, 36 water molecules could be found in the structure via Fourier and total OMIT maps. A subsequent single-crystal experiment (a crystal having finally been grown) confirmed the accuracy of the powder determination.
[8a] Combined Rietveld and stereochemical restraint refinement of a protein crystal structure. R. B. Von Dreele, J. Appl. Cryst., 32, 1084 (1999).
[8b] Second SH3 domain of Ponsin solved from powder diffraction. I. Margiolaki, J. P. Wright, M. Wilmanns, A. N. Fitch, and N. Pinotsis, J. Am. Chem. Soc., 129, 11865 (2007).
(top)
Human insulin
One of the practical aspects of the project is the fact that some therapeutic forms of proteins used medicinally are in the form of microcrystalline solids. There are many added benefits from using a crystalline form of a protein: it permits better control over the release rate by optimisation of the crystal size and polymorphism, and it should also offer an extended shelf life. Insulin is one such example of a treatment injected in microcrystalline form. Thus the study of the polymorphism of human insulin complexed with selected ligands is important for improving drug delivery for patients suffering from diabetes. Cocrystallization trials of human insulin with phenol or resorcinol as a function of pH (4 - 8.9) revealed a previously unknown polymorph that formed in the pH range 5 - 5.7, figure 9. Indexing (using the program Topas) indicated the symmetry to be monoclinic, space group P21, with a unit cell volume of »1.84 × 106 Å3, with a = 114.0228(8) Å, b = 335.43(3) Å, c = 49.211(6) Å, β = 101.531(8)° [9]. Only data of great accuracy can allow such a large structure to be indexed, (indeed initial attempts produced an erroneous answer). Note there is a dominant-zone problem, in that many of the low-angle peaks on which indexing depends are hk0 reflections. Note also that a sinβ » b/3 which does not help with issues of peak overlap.
Figure 9. Pawley fit to the new phase of insulin (space group P21) crystallised in the presence of phenol at pH 5.7, l = 1.29983(2) Å.
[9] Structural studies of human insulin cocrystallized with phenol or resorcinol via powder diffraction. F. Karavassili, A.E. Giannopoulou, E. Kotsiliti, L. Knight, M. Norrman, G. Schluckebier, L. Drube, A.N. Fitch, J.P. Wright and I. Margiolaki, Acta Cryst (2012). D68, 1632-1641 (2012).
(top)
Hexameric form of bovine insulin
In another study, a series of bovine insulin samples, obtained in the pH range 5.0–7.6, were identified to be in T6 hexameric form. Pawley analyses of the high resolution powder diffraction patterns showed pronounced pH- and radiation-induced anisotropic modifications to the lattice, which were exploited in a 14-data-set Rietveld refinement with the known structure (PDB code 2a3g) as starting model to obtain an average crystal structure over the pH range investigated. The effect of anisotropic lattice modifications is to change the degree of overlap between neighbouring peaks in different patterns which may allow their individual intensities to be better defined, thus increasing the overall information content compared to a single pattern. A new restraint strategy was adopted for the refinement in which each amino acid is represented by a flexible rigid body, thus requiring a smaller number of refinable parameters and restraints than with an individual-atom refinement, which has been the approach in the past combined with stereochemical restraints. 1542 stereochemical restraints were imposed to refine the positions of 800 protein atoms, two Zn atoms and 44 water molecules using experimental data in the resolution range 18.2 – 2.7 Å for all profiles [10].
[10] High-resolution powder X-ray data reveal the T6 hexameric form of bovine insulin I. Margiolaki, A. E. Giannopoulou, J. P. Wright, L. Knight, M. Norrman, G. Schluckebier, A. N. Fitch and R. B. Von Dreele, Acta Cryst. D69, 978-990 (2013).
(top)
Urate oxidase
Another example of finding a new polymorph and its subsequent partial characterisation concerns urate oxidase [11], which is prescribed (raspburicase) to eliminate uric acid produced by the breakdown products of dying cancer cells for patients undergoing chemotherapy. Precipitation in pure water led to a polymorph indexed as orthorhombic in space group P21212 with lattice parameters of a = 133.647(2) Å, b = 135.938(2) Å, c =78.9043(9) Å, (cell volume 1.43 × 106 Å3), Figure 15, (Collings et al., 2010). A comparable orthorhombic phase does occur in the protein data bank, code 1xxj, complexed with 5-amino 6-nitrouracil, but with rather different lattice parameters, a » 126 Å, b » 142 Å, c » 81 Å.
Figure 11. LeBail fit to urate oxidase (space group P21212), l = 1.3 Å.
[11] Polymorphism of microcrystalline urate oxidase from Aspergillus flavus.I. Collings, Y. Watier, M. Giffard, S. Dagogo, R. Kahn, F. Bonneté, J. P. Wright, A. N. Fitch and I. Margiolaki, Acta Cryst. D66, 539-548 (2010).
(top)
References:
[1] Charge order at the frontier between the molecular and solid states in Ba3NaRu2O9. Kimber S.A.J., Senn M.S., Fratini S., Wu H., Hill A.H., Manuel P., Attfield J.P., Argyriou D.N., Henry P.F. Phys. Rev. Lett. 108, 217205, (2012).
[2] Evidence for Electronic Phase Separation between Orbital Orderings in SmVO3. M. H. Sage, G. R. Blake, G. J. Nieuwenhuys, and T. T. M. Palstra, Phys. Rev. Lett., 96, 036401 (2006).
[3] Solving Larger Molecular Crystal Structures from Powder Diffraction Data by Exploiting Anisotropic Thermal Expansion. M. Brunelli, J. P. Wright, G. B. M. Vaughan, A. J. Mora, and A. N. Fitch, Angew. Chem. Int. Ed., 42, 2029 (2003).
[4] Complex zeolite structure solved by combining powder diffraction and electron microscopy. F. Gramm, C. Baerlocher, L. B. McCusker, S. J. Warrender, P. A. Wright, B. Han, S. B. Hong, Z. Liu, T. Ohsuna, and O. Terasaki, Nature, 444, 79–81 (2006).
[5] A highly crystalline microporous hybrid organic-inorganic aluminosilicate resembling the AFI-type zeolite. Bellussi G., Millini R., Montanari E., Carati A., Rizzo C., Parker Jr W.O., Cruciani G., De Angelis A., Bonoldi L., Zanardi S. Chem. Comm. 48, 7356, (2012).
[6a] A Hybrid Solid with Giant Pores Prepared by a Combination of Targeted Chemistry, Simulation, and Powder Diffraction. G. Férey, C. Serre, C. Mellot-Draznieks, F. Millange, S. Surblé, J. Dutour, and I. Margiolaki, Angew. Chem. Int. Ed., 43, 6296 (2004).
[6b] A Chromium Terephthalate-Based Solid with Unusually Large Pore Volumes and Surface Area. G. Férey, C. Mellot-Draznieks, C. Serre, F. Millange, J. Dutour, S. Surblé, and I. Margiolaki, Science, 309, 2040 (2005).
[7] A lithium-organic framework with coordinatively unsaturated metal sites that reversibly binds water. El Osta R., Frigoli M., Marrot J., Guillou N., Chevreau H., Walton R.I., Millange F. Chemical Communications 48, 10639,(2012).
[8a] Combined Rietveld and stereochemical restraint refinement of a protein crystal structure. R. B. Von Dreele, J. Appl. Cryst., 32, 1084 (1999).
[8b] Second SH3 domain of Ponsin solved from powder diffraction. I. Margiolaki, J. P. Wright, M. Wilmanns, A. N. Fitch, and N. Pinotsis, J. Am. Chem. Soc., 129, 11865 (2007).
[9] Structural studies of human insulin cocrystallized with phenol or resorcinol via powder diffraction. F. Karavassili, A.E. Giannopoulou, E. Kotsiliti, L. Knight, M. Norrman, G. Schluckebier, L. Drube, A.N. Fitch, J.P. Wright and I. Margiolaki, Acta Cryst (2012). D68, 1632-1641 (2012).
[10] High-resolution powder X-ray data reveal the T6 hexameric form of bovine insulin. I. Margiolaki, A. E. Giannopoulou, J. P. Wright, L. Knight, M. Norrman, G. Schluckebier, A. N. Fitch and R. B. Von Dreele, Acta Cryst. D69, 978-990 (2013).
[11] Polymorphism of microcrystalline urate oxidase from Aspergillus flavus.I. Collings, Y. Watier, M. Giffard, S. Dagogo, R. Kahn, F. Bonneté, J. P. Wright, A. N. Fitch and I. Margiolaki, Acta Cryst. D66, 539-548 (2010).