The TR-icOS setup: time-resolved microsecond UV-Vis absorption spectroscopy on protein crystals
An instrument to measure time-resolved UV–Vis absorption spectra from small protein crystals using a pump-probe scheme with microsecond-to-second delays has been developed at the icOS Lab as a preparative tool for time-resolved serial synchrotron crystallography experiments.
Time-resolved macromolecular crystallography (TR-MX) aims to determine the structure of intermediate states in the reaction of biomolecules such as photoreceptors or enzymes in order to help decipher their mechanism. Inspired by experiments performed at X-ray free electron lasers (XFELs), serial synchrotron crystallography (SSX) has been developed by using single diffraction images from a large number of micrometre-sized crystals, or ‘microcrystals’, paving the way for time-resolved SSX (TR-SSX).
The ESRF is the first fourth-generation synchrotron that has carried out the construction of a specific TR-SSX beamline, ID29, to take advantage of its high brilliance to produce large bandwidth monochromatic microsecond pulses. It makes possible, using pump-probe schemes (Figure 1a), the mechanistic study of photoactive proteins and other suitable systems with time resolutions down to microseconds. Photoreactions of proteins do not necessarily proceed in the crystalline state exactly as in solution; hence, it is highly desirable to perform spectroscopic experiments on crystalline samples prior to any TR-MX experiment to help validate the rise and decay of the various intermediates so that relevant time points can be chosen. Moreover, absorption of multiple photons from the pump laser could affect the photoreaction and potentially lead to artefacts in the interpretation of structural intermediates. Power titration spectroscopic experiments prior to the diffraction experiment should help identify these potential artefacts.
Click figure to enlarge
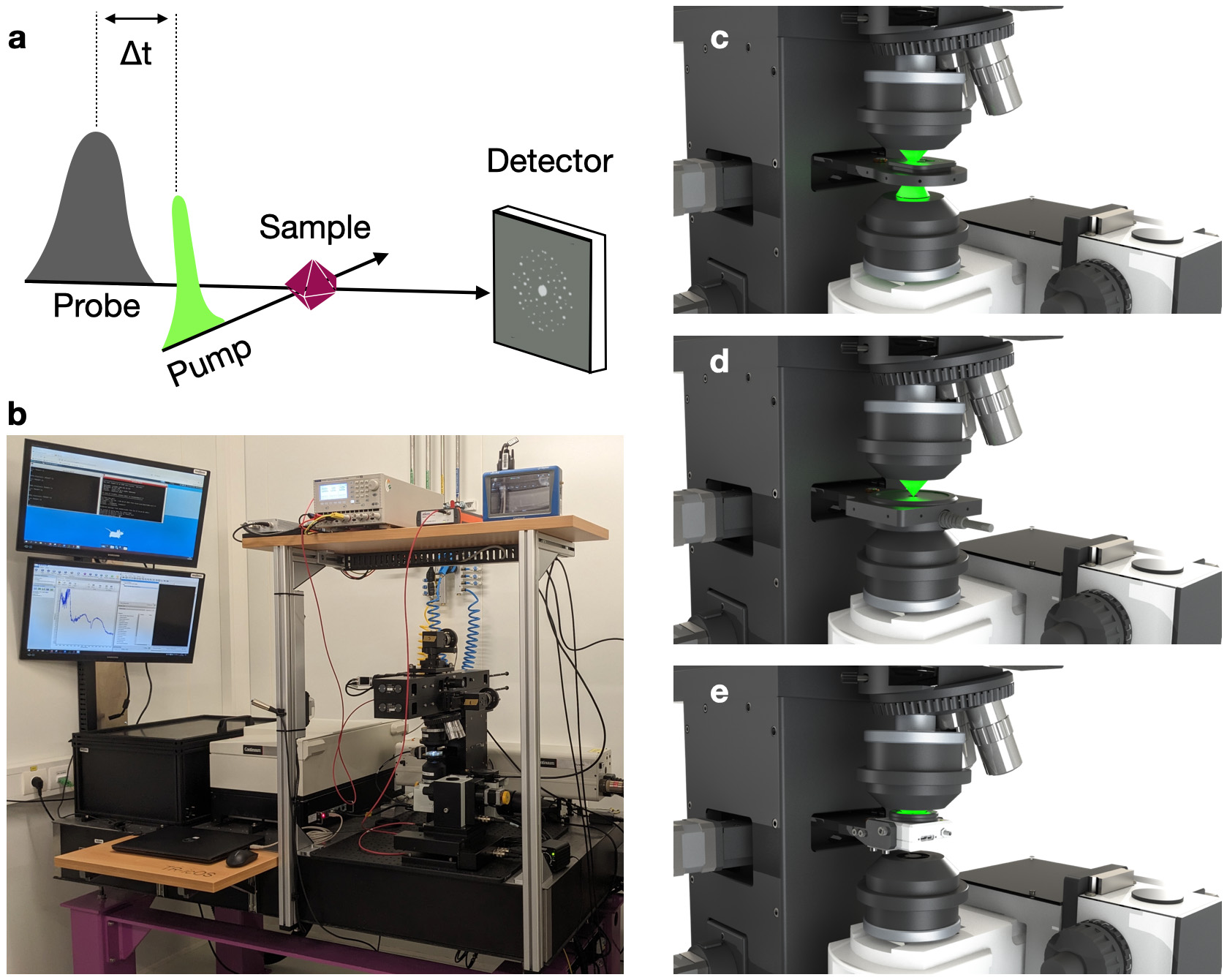
Fig. 1: a) Principle of a pump-probe spectroscopic or diffraction experiment. b) General view of the TR-icOS instrument. Close up on sample stage equipped with (c) sample holder, (d) laser energy measurement module and (e) beam profiling module.
In order to prepare and support TR-SSX experiments, a setup capable of measuring time-resolved UV-Vis absorption spectra on protein crystals on the microsecond to second time scale has been built: the TR-icOS instrument (TR-icOS stands for ‘time-resolved in crystallo Optical spectroscopy’) (Figure 1). It is accommodated within the icOS Lab, which is located between beamlines ID29 and ID30B. The core of the instrument is an opto-mechanical setup containing a motorised sample stage, on which crystals are placed within two plastic sheets preventing sample dehydration. The possibility of recording good signal-to-noise ratio spectra from a single crystal of size ranging from 10 to 40 microns in each direction is ensured by using two reflective focusing objectives, whose focal volumes are matched thanks to the motorisation of the lower objective. A tuneable nanosecond laser is used as the pump (possible wavelengths: 355 nm, and between 400 and 2750 nm) and a microsecond xenon flash lamp is used as the probe. The CITY board developed by the ESRF’s Electronics Unit ensures the synchronisation of all instruments, and controls in particular the delay time between the pump and the probe signals.
Click figure to enlarge
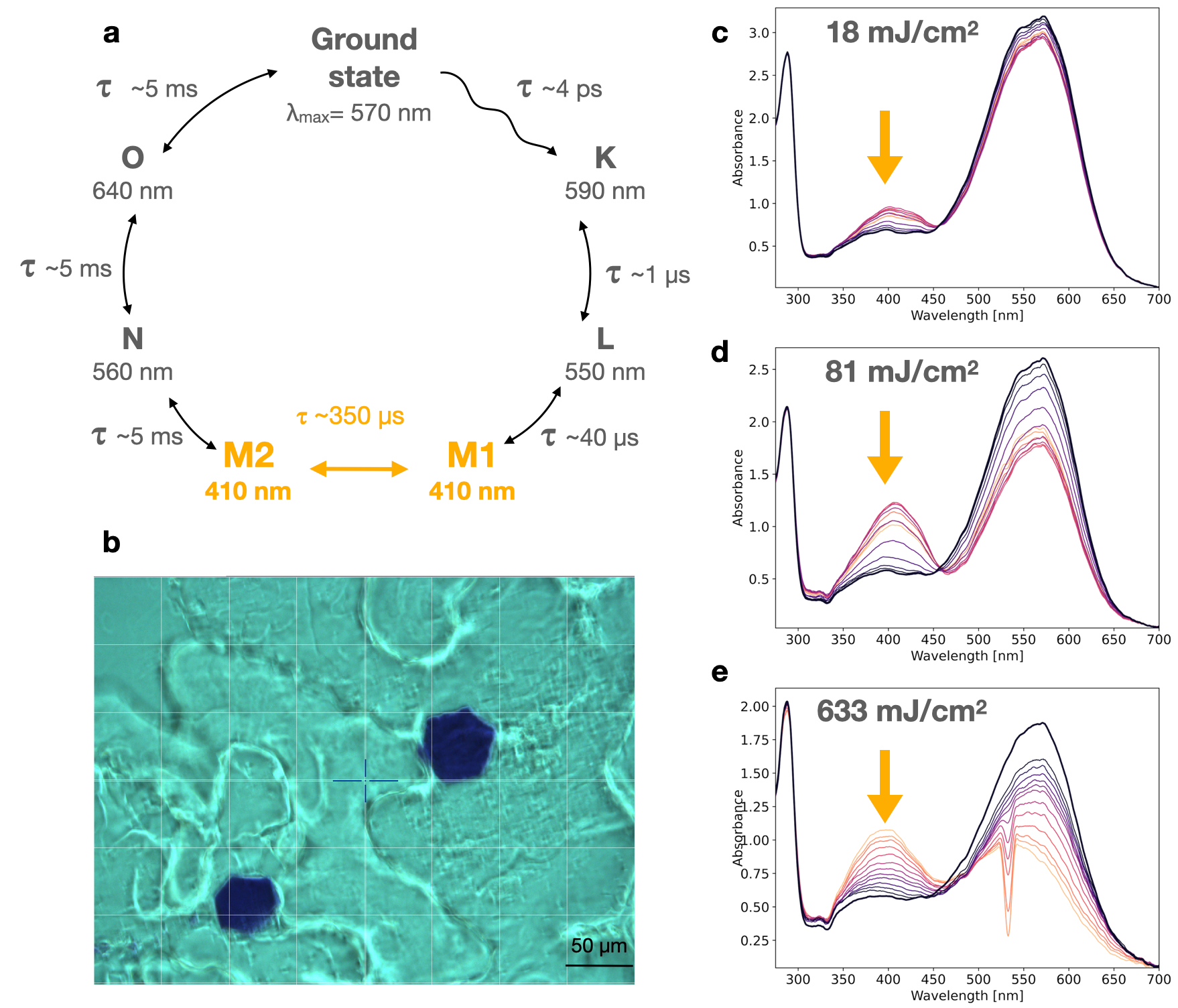
Fig. 2: Spectroscopic characterisation of the membrane protein bacteriorhodopsin in the crystalline state. a) Photocycle of bacteriorhodopsin, on which the M-state is highlighted in orange. b) Bacteriorhodopsin crystals photographed on the TR-icOS setup. c,d,e) Series of pump-probe spectra recorded between 3 ms and 1 s, with increasing light fluence.
Using the TR-icOS instrument, one can rapidly record pump-probe spectra from single crystals with time delays ranging from a few microseconds to seconds and beyond. This can be repeated at various laser pulse energies to track the potential presence of artefacts arising from two-photon absorption, which amounts to a power titration of a photoreaction. This approach was applied to monitor the rise and decay of the M-state in the photocycle of crystallised bacteriorhodopsin (Figure 2), and demonstrated that the photocycle is increasingly altered with laser pulses of peak fluence greater than 100 mJ/cm2, providing experimental laser and delay parameters for a successful TR-SSX experiment.
The TR-icOS instrument is unique for its capacity to record transient UV-Vis absorption spectra from single crystals, but can also be used for slurries of very small microcrystals. It provides a very handy tool for spectroscopic characterisation of optimal delay times and laser power before TR-SSX experiments.
Principal publication and authors
The TR-icOS setup at the ESRF: time-resolved microsecond UV-Vis absorption spectroscopy on protein crystals, S. Engilberge (a,b), N. Caramello (a,c), S. Bukhdruker (a,d,e), M. Byrdin (b), T. Giraud (a), P. Jacquet (b), D. Scortani (a), R. Biv (a), H. Gonzalez (a), A. Broquet (a), P. van der Linden (a,f), S.L. Rose (a), D. Flot (a), T. Balandin (d,e), V. Gordeliy (b,d,e), J.M. Lahey-Rudolph (g), M. Roessle (g), D. de Sanctis (a), G.A. Leonard (a), C. Mueller-Dieckmann (a), A. Royant (a,b), Acta Crystallogr. D, in press, https://doi.org/10.1107/S2059798323010483
(a) ESRF
(b) Univ. Grenoble Alpes, CNRS, CEA, Institut de Biologie Structurale (IBS), Grenoble (France)
(c) Hamburg Centre for Ultrafast Imaging, Universität Hamburg, Hamburg (Germany)
(d) Institute of Biological Information Processing (IBI-7: Structural Biochemistry), Forschungszentrum Jülich, Jülich (Germany)
(e) JuStruct: Jülich Center for Structural Biology, Forschungszentrum Jülich, Jülich (Germany)
(f) PSCM (Partnership for Soft Condensed Matter), ESRF
(g) Technische Hochschule Lübeck – University of Applied Sciences, Lübeck (Germany)