- Home
- News
- Spotlight on Science
- Cryo-electron microscopy...
Cryo-electron microscopy sheds light on heart regulation and cardiac diseases
20-07-2023
Scientists have used cryo-electron microscopy (cryo-EM) at beamline CM01 to solve the structure of the sequestered state of β-cardiac myosin, a key element in energy-saving and regulation within the heart. The structure allows to understand the development of inherited cardiac diseases.
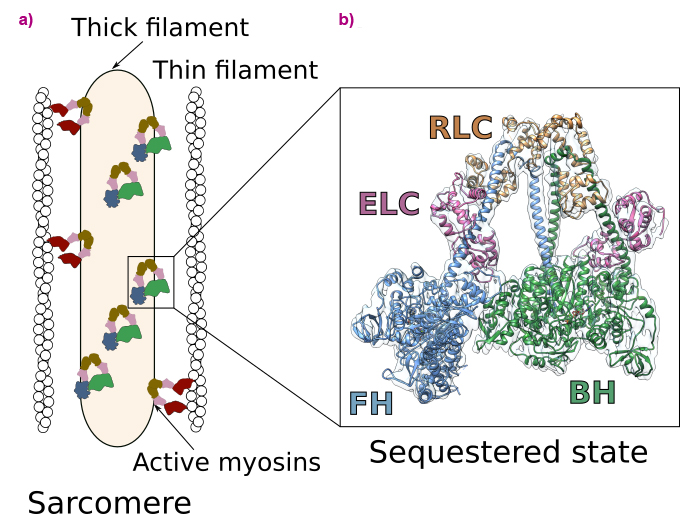
Heart contraction is a highly regulated process relying on a specific molecular motor: β-cardiac myosin. The filaments of β-cardiac myosin (thick filaments), along with actin filaments (thin filaments), form the sarcomere, which is the fundamental unit of the cardiac muscle (Figure 1a). During heart contraction, myosin heads interact with the actin filament, thus triggering the shortening of the sarcomeres [1]. Contraction is driven by ATP, the universal energy source of all living organisms, and is highly regulated. To avoid energy loss, a proportion of myosins adopts a folded-back, auto-inhibited conformation called the sequestered state (Figure 1a). These shut-down myosins can be converted into an on-state if exertion increases. Alteration of the stability of the sequestered state of β-cardiac myosin leads to serious diseases such as hypertrophic cardiomyopathies (HCM) [2].
Click image to enlarge
Fig. 1: Function and regulation of the cardiac sarcomere. a) Schematic view of the sarcomere. Myosins are organised in thick filaments that are close to thin filaments containing F-actin. Contraction and shortening of the sarcomere occurs when active myosin heads interact with F-actin in an ATP-dependent process. In a normal level of exertion, a proportion of myosin heads adopts an auto-inhibited sequestered state. b) High-resolution cryo-EM structure of the sequestered state of β-cardiac myosin. In this state, two heads interact with each other, shutting down the ability to use ATP and to interact with actin. The motif is asymmetric and comprises the blocked head (BH) and the free head (FH). Each myosin head binds two light chains (the essential light chain, ELC and the regulatory light chain, RLC).
Heart transplantation and pacemakers were the only treatments available for end-stage disease up to now due to the lack of understanding of the mechanism behind HCM. However, the development of small molecules able to modulate heart contraction by directly targeting β-cardiac myosin is now considered the most promising treatment against cardiac diseases. In 2022, the FDA approved the release of Mavacamten (CAMZYOSTM), a specific β-cardiac myosin inhibitor to treat occlusive HCM, and the drug is now available to patients. Interestingly, Mavacamten stabilises the sequestered state of β-cardiac myosin by an unknown mechanism of action. Studying the sequestered state of β-cardiac myosin is thus essential to decipher how these revolutionary treatments work against inherited cardiomyopathies.
In this work, cryo-electron microscopy was used at beamline CM01 to determine the high-resolution structure of human β-cardiac myosin in the sequestered state (Figure 1b). The structure reveals the details of the interfaces stabilising the double-headed, folded-back conformation and discloses the mechanism of auto-inhibition of β-cardiac myosin. This structure allows to map the location of HCM-causing point mutations, demonstrating how they destabilise the sequestered state. Thus, it shows that most of these mutations cause hypercontractility by increasing the number of myosin heads involved in force production. The successful determination of the structure was made possible through meticulous screening of various experimental conditions and rigorous image analysis of the high-quality dataset acquired using the Titan Krios microscope at CM01.
The results shed light on the development of inherited cardiomyopathies and open new avenues for the design of personalised medicine approaches that will be essential to treat these diseases. The next steps will be to study precisely how small molecules such as Mavacamten can stabilise the sequestered state of β-cardiac myosin.
Principal publication and authors
Cryo-EM structure of the folded-back state of human β-cardiac myosin, A. Grinzato (a), D. Auguin (b,c), C. Kikuti (b), N. Nandwani (d), D. Moussaoui (a), D. Pathak (d), E. Kandiah (a), K.M. Ruppel (d,e), J.A. Spudich (d), A. Houdusse (b), J. Robert-Paganin (b), Nat. Commun. 14(1), 3166 (2023); https://doi.org/10.1038/s41467-023-38698-w.
(a) ESRF
(b) Structural Motility, Institut Curie, Paris Université Sciences et Lettres, Sorbonne Université, CNRS UMR144, Paris (France)
(c) Laboratoire de Biologie des Ligneux et des Grandes Cultures, Université d'Orléans, UPRES EA 1207, INRA-USC1328, Orléans (France)
(d) Department of Biochemistry, Stanford University School of Medicine, CA (USA)
(e) Department of Pediatrics, Stanford University School of Medicine, CA (USA)
References
[1] J. Robert-Paganin et al., Chem Rev. 120(1), 5-35 (2020).
[2] J. Robert-Paganin et al., Nat Commun. 9(1), 4019 (2018).
| About the beamline: CM01 |
|
Beamline CM01 hosts the ESRF’s Titan Krios cryo-electron microscope (cryo-EM) for single particle experiments. It is equipped with a K3 direct electron detector, a Quantum LS imaging filter and a Volta phase plate. At the ESRF, Cryo-EM is used as a complementary technique to macromolecular crystallography and BioSAXS. Cryo-EM can be used for protein structure determination with near atomic resolution by single-particle imaging to obtain subnanometre-resolution structures of up to ~2 Å including protein complexes and viruses with sizes typically of 150 kDa and above. It is commonly used for larger proteins and complexes that are intrinsically difficult to crystallise. Cryo-EM image analysis is powerful enough to enable in silico classification of different conformation states of the sample if they co-exist in solution. |