- Home
- News
- Spotlight on Science
- Cryo-electron microscopy...
Cryo-electron microscopy and SAXS help reveal how the sleeping sickness parasite evades the human innate immune system
05-06-2023
Cryo-electron microscopy and small-angle X-ray scattering at beamline BM29 have been used to determine the structures and conformations of a surface protein that helps human-infective trypanosomes to evade the alternative pathway of the innate immune response.
Share
Researchers from the Institute of Organic Chemistry and Biochemistry of the Czech Academy of Sciences in Prague have made a significant breakthrough in understanding how the parasite Trypanosoma brucei gambiense, which causes African sleeping sickness, evades the human innate immune system. A new study has revealed the structure and conformational dynamics of the parasite's invariant surface glycoprotein 65 (ISG65) in complex with human complement factors C3 and C3b, key components of the immune system's alternative pathway. This new insight could lead to the development of new treatments for African sleeping sickness, which affects thousands of people in sub-Saharan Africa.
African sleeping sickness is caused by the parasite T. brucei gambiense, which is transmitted by the tsetse fly. The disease can be fatal if left untreated, and currently there are no effective vaccines or drugs available to prevent or treat the disease. One of the ways the parasite is able to evade the immune system is by expressing a dense surface layer of variant surface glycoproteins (VSGs) that undergo constant antigenic variation, preventing the host from mounting a long-lasting immune response. However, another class of surface proteins, the invariant surface glycoproteins (ISGs), remain relatively constant and do not trigger immune detection of the parasite. The biology of these proteins remains poorly understood, but they are believed to play a crucial role in the parasite's ability to evade the immune system.
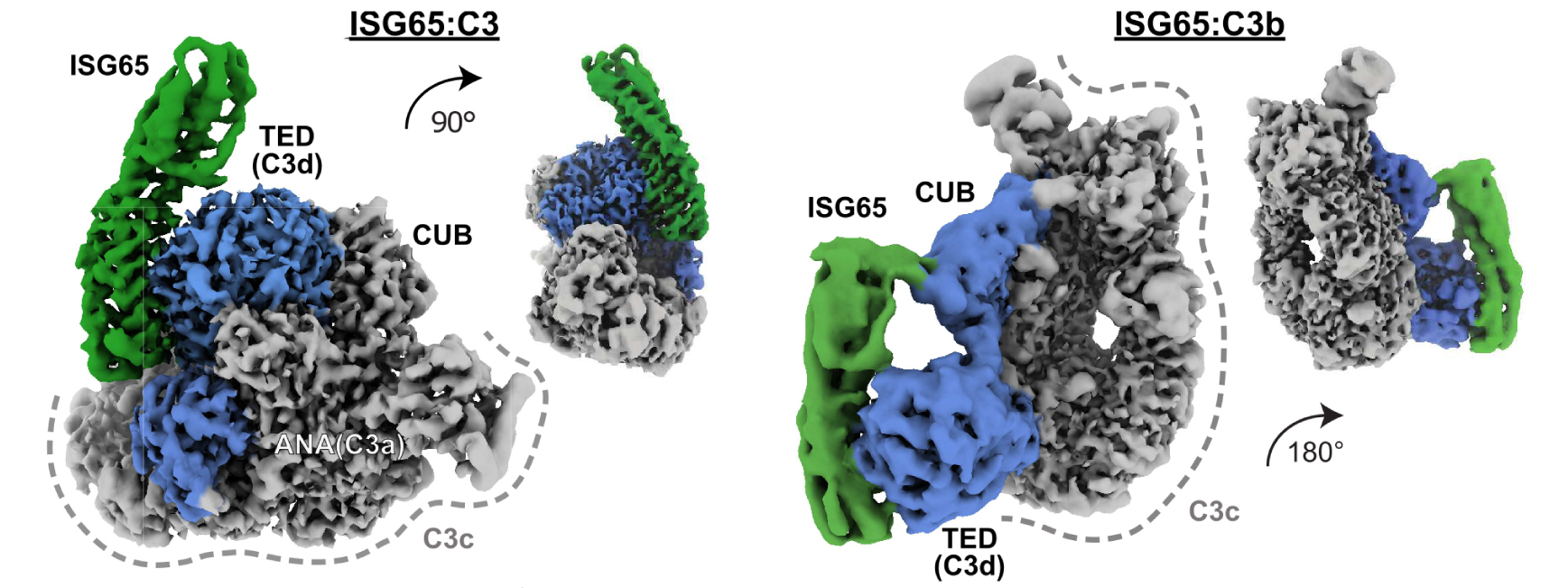
In this study, researchers used cryo-electron microscopy (cryo-EM) at CEITEC (Czech Republic) and SOLARIS (Poland) to capture detailed images of ISG65 bound to human complement C3 and C3b (Figure 1). They found that ISG65 is able to specifically inhibit the C5 convertase of the alternative complement pathway, thereby preventing the immune system from attacking the parasite at an early stage of infection.
Click image to enlarge
Fig. 1: Cryo-EM structures of T. brucei gambiense ISG65 in complex with native C3 and C3b. Cryo-EM density maps showing side views of ISG65:C3 (left), and ISG65:C3b (right) at two different angles. ISG65 is represented by map regions coloured in green. Interacting domains in C3 and C3b are depicted in blue. In both C3 conformations, the thioester domain (TED) provides the primary interface. ISG65 and TED (C3d, when liberated) show a high degree of shape complementarity. Smaller, secondary interfaces are located in the anaphylatoxin (ANA) domain (native C3) and CUB (C3b). The remaining scaffold (C3c, grey) shows no additional contact points.
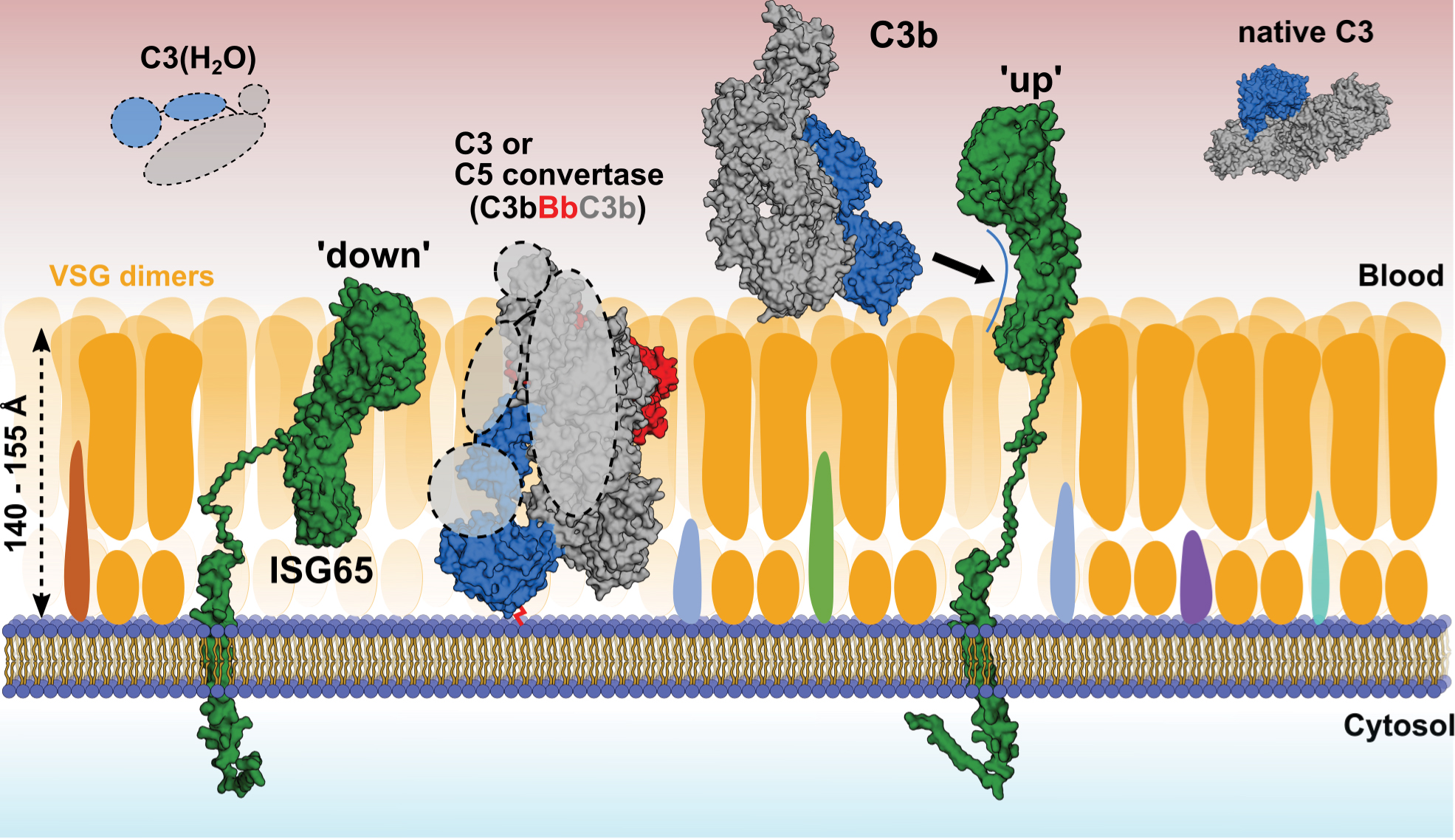
Using small-angle X-ray scattering (SAXS) at beamline BM29, the researchers also showed that ISG65 can exist in two different states: one where it would extend away from the cell surface of the parasite and bind complement factor C3b, and another where it would be embedded in the protective surface layer of the parasite (Figure 2). This flexible connection to the cell surface allows ISG65 to switch between these two states, likely enabling the parasite to avoid detection by the host's immune system while still being able to bind and remove complement factor C3b from the surface.
Click image to enlarge
Fig. 2: Model of complement binding on the surface of T. brucei gambiense. Using SAXS and ensemble optimisation, conformations of the disordered C-terminal linker of ISG65 were calculated in solution. In its compact ‘down’ position, ISG65 would remain embedded within the VSG coat, with its C3-binding epitopes being concealed and only the disordered head domain exposed to the outside. In this state, ISG65 may bind and inactivate AP convertases via C3b that may pass through the VSG coat, where it could covalently bind to the plasma membrane via Gln1013 of the former thioester (red connecting line). In its extended ‘up’ position, ISG65 would be to able intercept C3b outside the VSG coat and transport it to the flagellar pocket for uptake and subsequent lysosomal degradation. C3b would hereby either be present in close proximity to the coat or attached covalently or non-covalently to VSGs. C3(H2O) and native C3 are depicted in the solution, as it is currently unknown whether they bind to the trypanosome surface. Surface receptors embedded within the VSG coat are depicted in different colours. The atomic structures of C3(H2O) and the AP C5 convertase have not been determined and are therefore shown as dashed lines.
The findings of this study provide new insights into how trypanosomes interact with host factors in the bloodstream, adding another layer of complexity to our understanding of these parasites. The implications of the findings extend beyond trypanosome biology to our understanding of the immune system and its interactions with pathogens. While the complexity of the immune system can make it difficult to study, the integration of structural biology can offer unprecedented insights into these interactions, paving the way for a better understanding of immune defence mechanisms.
Principal publication and authors
Cryo-EM structures of Trypanosoma brucei gambiense ISG65 with human complement C3 and C3b and their roles in alternative pathway restriction, H. Sülzen (a), J. Began (a), A. Dhillon (a), S. Kereiche (a), P. Pompach (b), J. Votrubova (a), F. Zahedifard (c), A. Subrtova (a), M. Safner (a), M. Hubalek (a), M. Thompson (a), M. Zoltner (c), S. Zoll (a), Nat. Commun. 14, 2403 (2023); https://doi.org/10.1038/s41467-023-37988-7
(a) Institute of Organic Chemistry and Biochemistry of the Czech Academy of Sciences, Prague (Czech Republic)
(b) Institute of Biotechnology of the Czech Academy of Sciences, Vestec (Czech Republic)
(c) Department of Parasitology, Faculty of Science, Charles University Prague, Biocev, Vestec (Czech Republic)
| About the beamline: BM29 |
| BM29 BioSAXS is a tuneable energy beamline (from 7 to 15 keV) for small-angle X-ray scattering (SAXS) experiments of biological macromolecule solutions, with the goal to determine their 3D structures in a natural state with a 'low' resolution (~nm). Samples including proteins, nucleic acids, protein-based complexes, lipids, membrane proteins, synthetic polymers, glycoproteins, viruses, etc., can be investigated under various conditions (temperature, buffer, pH, kinetics) in a high-throughput manner, or a HPLC unit can be used for in-situ (online) purification. This high-performance beamline has been designed to be easy to set up and user-friendly, with an automated data collection and processing pipeline. |