- Home
- News
- Spotlight on Science
- Better together:...
Better together: a novel bimetallic catalyst for the efficient processing of biomass
21-05-2015
Chemists from Utrecht University in the Netherlands, together with researchers at University College London (UK) and Lehigh University (USA), have managed to develop a novel bimetal alloy catalyst which is both faster and more efficient when performing a crucial intermediate catalytic stage in the processing of biomass into valuable renewable products such as plastics and fuels. These results were recently reported in the journal Nature Communications, which also details how extended X-ray absorption fine structure (EXAFS) measurements performed at the ESRF played a crucial role in determining the nature and location of the catalytically active sites.
Share
By 2050, some three-quarters of the world’s population will be living in cities. The growing number of cities with over a million inhabitants is demanding more sustainable and closed carbon loops for a good living environment. Chemical building blocks derived from biomass can form a significant contribution to the increasing demand for sustainable materials and fuels. In this case it concerns non-edible and fast-growing biomass such as straw, foliage and wood. During the processing of biomass, a select number of chemical building blocks form that serve as the basis for the production of a wide range of renewable chemicals such as plastics, fuels and solvents. To obtain these intermediate products in high yield and to ensure their proper conversion, catalysts are crucial.
Levulinic acid is one of the most important intermediary products of the processing of cellulose, a component of plant material. By means of catalytic conversion, this product is transformed into a different ‘green’ building block known as γ-valerolactone. The catalysts used for this conversion are often supported metals such as gold nanoparticles on a titanium dioxide support (Au/TiO2). In our recent report, we experimented with using combinations of metals such as Au, Pd, Ru on TiO2 and which are known to readily alloy in order to tune the structure/electronic properties of these bimetallic nanoparticles to bring about a much quicker and more efficient process of converting biomass-derived levulinic acid into γ-valerolactone. In particular, we found that one of the alloys, Ru-Pd, brought about the desired conversion both very quickly and very specifically [1-3].
Control of the particle size and metal distribution is critical for the development of these optimally performing catalysts and a combination of Fourier-transform infrared (FTIR), X-ray photoelectron spectroscopy (XPS) and scanning transmission electron microscopy (STEM) in combination with EXAFS was used to determine this. But it was the application of EXAFS in particular, recorded at both BM26A and BM23 beamlines and at both edges in a bimetal species, that allowed a detailed structural model of the active catalyst to be obtained (Figure 1). From these results, we were able to conclude that the active nanoparticles are some 1.5 nm in size and form random alloys and, furthermore, that the key to super-efficiency in Ru-Pd was a high degree of Ru dispersion in the Pd matrix [2].
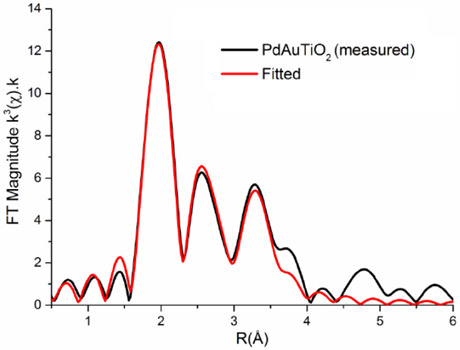 |
|
Figure 1. Pd K-edge Fourier transformed EXAFS data for bimetallic Pd-Au on TiO2 with a random alloy structure. |
Principal publication and authors
W. Luo (a), M. Sankar (a), A.M. Beale (a,b,c), Q. He (d), C.J. Kiely (d), P.C.A. Bruijnincx (a), B.M. Weckhuysen (a), High performing and stable supported nano-alloys for the catalytic hydrogenation of levulinic acid to γ-valerolactone, Nature Communications 6, 6540 (2015).
(a) Inorganic Chemistry and Catalysis, Debye Institute for Nanomaterials Science, Utrecht University (The Netherlands)
(b) UK Catalysis Hub, Research Complex at Harwell, Rutherford Appleton Laboratory, Oxfordshire (UK)
(c) Department of Chemistry, University College London (UK)
(d) Department of Materials Science and Engineering, Lehigh University, Bethlehem (USA)
References
[1] M. Sankar, N. Dimitratos, P.J. Miedziak, P.P. Wells, C.J. Kiely, G. Hutchings, Chem. Soc. Rev. 41, 8099 (2012).
[2] W. Luo, M. Sankar, A.M. Beale, Q. He, C.J. Kiely, P.C.A. Bruijnincx, B.M. Weckhuysen, Nature Commun. 6, 6540 (2015).
[3] W. Luo, M. Sankar, P.C.A. Bruijnincx, B.M. Weckhuysen, Patent application PCT/NL2014/050569.
Top image: An active Ru-Pd bimetallic particle for the selective conversion of levulinic acid to γ-valerolactone.



