Paintings
Identification and composition of pigments, driers, gilding materials, binders and their mixture in paints
The ID21 instruments are regularly exploited to determine the composition of paint fragments. This can be to identify the composition of pigments, being for example lead white which has been extensively used since Antiquity until the 20th Century, or chrome yellow, which was invented in the 19th Century. In both cases, analyses aim at identifying the pigment composition and at correlating it with manufacturing recipes described in historical texts. This combined analysis can give clues about the historical evolution of recipes, aiming for example at reducing cost, improving photo-stability, etc. Experiments are usually carried out concurrently on fragments sampled in historical paintings and on model samples, obtained following historical recipes.
In addition of the analysis of the pigments themselves, the ID21 microscopes have also been used to identify organic binders (and in particular revealed the use of oil in Bamiyan Buddhist wall paintings) as well as to characterize products of interaction of pigments, driers and organic binding media.
 |
V. Gonzalez, G. Wallez, E. Ravaud, M. Eveno, I. Fazlic, T. Fabris, A. Nevin, T. Calligaro, M. Menu, V. Delieuvin, and M. Cotte, "X-ray and Infrared Microanalyses of Mona Lisa’s Ground Layer and Significance Regarding Leonardo da Vinci’s Palette", Journal of the American Chemical Society, 145 (42) 23205–23213 (2023)
|
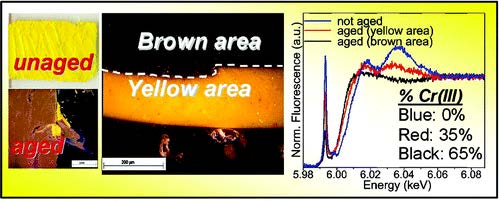 |
L. Monico, G. Van der Snickt, K. Janssens, W. De Nolf, C. Miliani, J. Verbeeck, H. Tian, H. Tan, J. Dik, M. Radepont and M. Cotte, "Degradation Process of Lead Chromate in Paintings by Vincent van Gogh Studied by Means of Synchrotron X-ray Spectromicroscopy and Related Methods. 1. Artificially Aged Model Samples", Analytical Chemistry, 83, 1214-1223 (2011). |
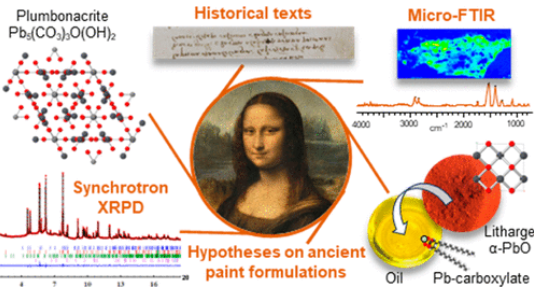
An exceptional microsample from the ground layer of Leonardo da Vinci’s Mona Lisa was analyzed by high-angular resolution synchrotron X-ray diffraction and micro Fourier transform infrared spectroscopy, revealing a singular mixture of strongly saponified oil with high lead content and a cerussite (PbCO3)-depleted lead white pigment. The most remarkable signature in the sample is the presence of plumbonacrite (Pb5(CO3)3O(OH)2), a rare compound that is stable only in an alkaline environment. Leonardo probably endeavored to prepare a thick paint suitable for covering the wooden panel of the Mona Lisa by treating the oil with a high load of lead II oxide, PbO. The review of Leonardo’s manuscripts (original and latter translation) to track the mention of PbO gives ambiguous information. Conversely, the analysis of fragments from the Last Supper confirms that not only PbO was part of Leonardo’s palette, through the detection of both litharge (α-PbO) and massicot (β-PbO) but also plumbonacrite and shannonite (Pb2OCO3), the latter phase being detected for the first time in a historical painting.
 |
A. Kostomitsopoulou Marketou, F. Giannici, S. Handberg, W. de Nolf, M. Cotte, and F. Caruso , "Synchrotron Radiation-Based Micro-XANES and Micro-XRF Study of Unsuccessfully Produced Egyptian Blue from the Late Hellenistic Production Site of Kos (Dodecanese, Greece)" Analytical Chemistry, 93 (33), 11557-11567 (2021) |
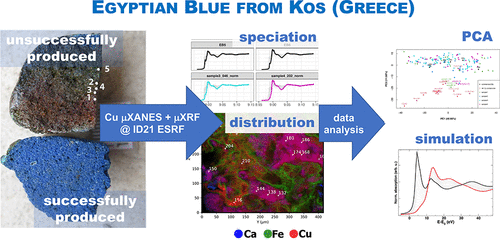
This paper examines the production technology of Egyptian blue, an ancient artificial pigment, through the investigation of an unsuccessfully produced pellet derived from the Hellenistic production site of Kos (Dodecanese, Greece). This heterogeneous material was investigated by a combination of laboratory and synchrotron radiation-based (SR) techniques: scanning electron microscopy coupled with energy-dispersive X-ray spectrometry, micro-Raman spectroscopy, high-resolution SR micro-X-ray fluorescence spectroscopy, and SR micro-X-ray absorption near-edge structure spectroscopy (XANES), at the ID21 beamline of the European Synchrotron Radiation Facility. Principal component analysis of a large dataset of 171 micro-XANES spectra acquired on the archaeological samples and on a series of reference copper compounds emphasizes high variations of XANES features due to different speciation and also orientation effects, as demonstrated by the simulated XANES spectra. The results indicate that, rather than inadequate firing temperatures that could have led to the reddish cuprite (Cu2O), unsuccessful production may occur due to the use of inappropriate starting materials, which contain an unusually high iron content. The contextual interpretation underlines the intertwined relationship between the production of Egyptian blue and metallurgy.
|
A. A. Gambardella, M. Cotte, W. de Nolf, K. Schnetz, R. Erdmann, R. van Elsas, V. Gonzalez, A. Wallert, P. D. Iedema, M. Eveno and K. Keune, "Sulfur K-edge micro- and full-field XANES identify marker for preparation method of ultramarine pigment from lapis lazuli in historical paints", Science Advances, 6, eaay8782 (2020). |
Ultramarine blue pigment, one of the most valued natural artist’s pigments, historically was prepared from lapis lazuli rock following various treatments; however, little is understood about why or how to distinguish such a posteriori on paintings. X-ray absorption near-edge structure spectroscopy at the sulfur K-edge in microbeam and full-field modes (analyzed with nonnegative matrix factorization) is used to monitor the changes in the sulfur species within lazurite following one such historically relevant treatment: heating of lapis lazuli before extracting lazurite. Sulfur signatures in lazurite show dependence on the heat treatment of lapis lazuli from which it is derived. Peaks attributed to contributions from the trisulfur radical—responsible for the blue color of lazurite—increase in relative intensity with heat treatment paralleled by an intensified blue hue. Matching spectra were identified on lazurite particles from five historical paint samples, providing a marker for artists’ pigments that had been extracted from heat-treated lapis lazuli.
 |
A. van Loon, A. A. Gambardella, V. Gonzalez, M. Cotte, W. De Nolf, K. Keune, E. Leonhardt, S. de Groot, A. N. Proaño Gaibor and A. Vandivere, "Out of the blue: Vermeer’s use of ultramarine in Girl with a Pearl Earring", Heritage Science, 8, 19 (2020). |
Johannes Vermeer (1632–1675) is known for his brilliant blue colours, and his frequent use of the costly natural ultramarine. This paper reveals new findings about ultramarine in the headscarf of Girl with a Pearl Earring (c. 1665, Mauritshuis). The painting was examined using a range of micro- and macroscale techniques as part of the Girl in the Spotlight research project (2018). Analysis of micro-samples mounted as cross-sections using SEM–EDX and FTIR-ATR showed that Vermeer used high-quality ultramarine in the blue headscarf, based on the relative abundance of bright blue particles of lazurite. Analysis with synchrotron sulphur K-edge XANES suggested that the ultramarine pigment was prepared—at least in part—from a heat-treated lapis lazuli rock. The entire painting was imaged using MS-IRR, MA-XRF, RIS, and digital microscopy to reveal the distribution of materials of the headscarf, and to give more insight into Vermeer’s painting process. The shadow part of the headscarf has a remarkably patchy appearance, due to paint degradation that is probably related to the large amounts of chalk Vermeer mixed in the ultramarine paint in this area. The question was raised as to whether extra chalk was added deliberately to the paint to adjust the handling properties or opacity, or whether the chalk was the substrate of a—now faded—yellow lake. Schematic paint reconstructions were made to investigate the effect of the addition of chalk or yellow lake on the paint properties. The analyses and reconstructions led to the hypothesis that the blue headscarf originally contained a wider range of different blue colour shades: an opaque light blue for the left (lit) zone, a slightly brighter opaque blue for the middle zone, and a deep dark blue-green glaze with alternating blue-green glazing brushstrokes for the shadow zone—now largely compromised by paint degradation.
 |
V. Gonzalez, M. Cotte, G. Wallez, A. van Loon, W. de Nolf, M. Eveno, K. Keune, P. Noble and J. Dik, "Identification of Unusual Plumbonacrite in Rembrandt's Impasto by Using Multimodal Synchrotron X-ray Diffraction Spectroscopy", Angewandte Chemie International Edition, 58 (2019). (Experiments carried out at ID13 and ID22). |
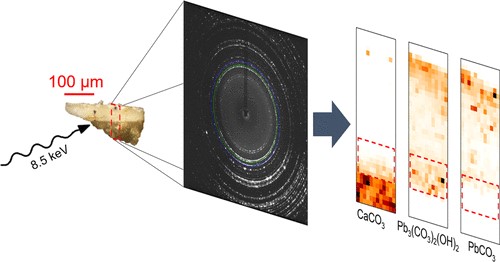
Rembrandt (1606–1669) is renowned for his impasto technique, involving his use of lead white paint with outstanding rheological properties. This paint was obtained by combining lead white pigment (a mixture of cerussite PbCO3 and hydrocerussite Pb3(CO3)2(OH)2) with an organic binding medium, but the exact formulation used by Rembrandt remains a mystery. A powerful combination of high‐angle and high‐lateral resolution x‐ray diffraction was used to investigate several microscopic paint samples from four Rembrandt masterpieces. A rare lead compound, plumbonacrite (Pb5(CO3)3O(OH)2), was detected in areas of impasto. This can be considered a fingerprint of Rembrandt's recipe and is evidence of the use of an alkaline binding medium, which sheds a new light on Rembrandt's pictorial technique.
 |
V. Gonzalez, G. Wallez, T. Calligaro, M. Cotte, W. De Nolf, M. Eveno, E. Ravaud and M. Menu, "Synchrotron-Based High Angle Resolution and High Lateral Resolution X-ray Diffraction: Revealing Lead White Pigment Qualities in Old Masters Paintings", Analytical Chemistry 2017, 89 (24), pp 13203–13211 |
Microsamples collected on 27 major paintings by Old European Masters dating from the 14th to the late 19th centuries were analyzed using synchrotron-based X-ray diffraction. Two complementary analytical configurations were used at beamlines ID22 (high angle resolution) and ID21 (high lateral resolution), in order to highlight markers of the different grades of the lead white pigments (mixture of cerussite PbCO3 and hydrocerussite Pb3(CO3)2(OH)2). Rietveld analysis and crystalline phases mapping at the microscale revealed the composition and microstructure of the pigments, shedding light on the preparation recipes and pigment choices of the artists through History.
 |
M. Cotte, E. Checroun, W. De Nolf, Y. Taniguchi, L. De Viguerie, M. Burghammer, P. Walter, C. Rivard, M. Salomé, K. Janssens and J. Susini, "Lead soaps in paintings: Friends or foes?", Studies in Conservation, 62, 2-23 (2017). |
The origin(s) and role(s) of metal soaps in paints are a worldwide concern today. These hybrid compounds, containing both fatty acid chains and metals associated with a carboxylate function, are increasingly identified in paints. As reviewed in the first part of this work, the presence of metal soaps in paints is differently interpreted in scientific publications: metal soaps are sometimes considered to play a positive role as anchor points, during paint drying processes; they can also be considered as responsible for many degradation processes (protrusions, efflorescences, darkening, etc.). Their origins are also interpreted in various ways. In some paintings (in particular from the twentieth century), they have sometimes introduced on purpose, as additives, to modify the physical properties of the painting materials. In older paintings, metal soaps are usually thought to result from an uncontrolled reaction of oil with lead-based pigments, in particular lead white, red lead, and lead tin yellow. In the second part of this work, the review of historical recipes of lead-based paint shows an important number of recipes based on controlled mixing of oil with lead driers. In the third part, the experimental reproduction of such traditional recipes using walnut oil and litharge (PbO) shows that lead soaps can be formed, both in about one hour at ∼100°C, or in about one month at room temperature. It shows as well that after a few years, litharge is no longer detected in the paint medium, while different lead carbonates are. Finally, the micro-infrared spectroscopy and micro-X-ray diffraction re-analysis of protrusions from a nine-year model painting shows together with lead soaps, the presence of Pb5(CO3)3(OH)2O (‘synthetic plumbonacrite’), an unusual phase recently observed in a protrusion from a painting by Vincent Van Gogh. This work highlights (i) the multiple origins and roles of metal soaps in paints and (ii) the importance of combining the analysis of fragments from historical paintings with the analysis and reproduction of historical recipes. In particular, we show that the components detected today in historical paintings may severely differ from those originally used or prepared by the painter, complicating the assessment of the painter's intentions. More than the presence of metal soaps, the key questions to be tackled should be about their origins and (re)mobilization.
 |
V. Otero, J. Vas Pinto, L. Carlyle, M. Vilarigues, M. Cotte and M. J. Melo, "19th Century chrome yellow and chrome deep from Winsor & Newton, M. A.", Studies in Conservation, 62, 123-149 (2017). |
The Winsor & Newton TM (W&N) nineteenth century archive database includes digitised images of hand-written instructions and workshop notes for the manufacture of their artists’ materials. For the first time, all 183 production records for yellow lead chromate pigments were studied and evaluated. They revealed that W&N produced essentially three pigment types: lemon/pale based on mixed crystals of lead chromate and lead sulphate [Pb(Cr,S)O4]; middle on pure monoclinic lead chromate [PbCrO4]; and deep that contains the latter admixed with basic lead chromate [Pb2CrO5]; accounting for 53, 22, and 21% of the production, respectively. Production records for primrose (4%) were also included since the formulation results in mixed crystals with a high percentage of lead sulphate, which, according to the literature, leaves it more prone to degradation. Each pigment type is characterised by only one or two main synthetic pathways; process variations reveal a systematic and thorough search for a high-quality durable product. A comparison of the chemical composition of pigment reconstructions with early W&N oil paint tubes showed that their records entitled ‘pale’ and ‘lemon’ correlated with the pigment in their tube labelled chrome yellow and, ‘middle’ and ‘deep’ with the label chrome deep. Lemon and middle pigment formulations were made into oil paints to assess their relative photo-stability. The degradation process was followed by colorimetry and was studied by synchrotron radiation-based techniques. Based on the X-ray absorption spectroscopy data, the possibility for creating a stability index for chrome yellows is discussed.
 |
M. Chaouche, X. X. Gao, M. Cyr, M. Cotte and L. Frouin, "On the origin of the blue/green color of blast-furnace slag-based materials: Sulfur K-edge XANES investigation", Journal of the American Ceramic Society, 100, 1707-1716 (2017). |
Slag‐based materials including mortars, concretes, Ca‐geopolymers, etc., are known to display a fascinating blue/green color upon hydration. This specific color is of particular concern in applications where visual esthetics are important. Yet only limited studies have been devoted to this phenomenon so far and its origin remains unexplained. It has sometimes been attributed, without any experimental evidence, to the presence of polysulfur species in the slag. To address the origin of this coloration, sulfur K‐edge X‐ray absorption near edge structure (XANES) spectroscopy was used to investigate the evolution of the speciation of sulfur during slag hydration. Three methods of slag activation were considered: thermal, portland cement, and sodium silicate. The impact of the activation method on the sulfur K‐edge XANES spectrum was examined first. Then, a comparison was made between the XANES of blue and white samples or zones with or without the blue color within the same sample. Independently of the activation route, the blue color was found to be unambiguously related to the presence of a prepeak in the corresponding XANES spectrum. This feature was absent for white samples or white zones. The prepeak, which was located at lower energy than the peak corresponding to the most common reduced sulfur species, was attributed to the presence of the trisulfur radical anion S3−. This blue chromophore is known to be at the origin of the deep blue color of the stone lapis lazuli or the ultramarine pigment (derived from lazurite).
|
Lluveras-Tenorio, I. Bonaduce, F. Sabatini, I. Degano, C. Blaensdorf, E. Pouyet, M. Cotte, L. Ma and M. P. Colombini, "The organic materials in the Five Northern Provinces’ Assembly Hall: disclosing the painting technique of the Qing dynasty painters in civil buildings", Applied Physics A, 1-11 (2015). |
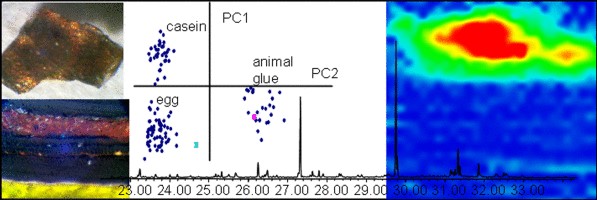
The beiwusheng huiguan (‘Meeting hall of the Five Northern Dynasties’) is a building complex from the Qing dynasty (1636–1912 ad) located in Wafangdian, near Ziyang, in the south of the Chinese Province of Shaanxi. Two of the preserved halls are richly decorated with wall paintings dated probably in 1848 ad and representing scenes of the ‘Romance of the Three Kingdoms’ and Confucian moral tales. They are a rare example of well-preserved mural paintings of high artistic value inside civil buildings. The aims of this paper are the chemical characterization and localization of organic materials used as binders and colorants in the wall paintings. A multi-analytical approach, consisting in the combined use of gas chromatographic–mass spectrometric techniques (GC/MS and Py-GC/MS) and high-pressure liquid chromatography with diode array detector (HPLC–DAD), was chosen for these purposes. Proteinaceous materials (animal glue and egg), saccharide material (fruit tree gum) and a siccative oil were identified in different paint layers supplying invaluable information about the painting technique used. Moreover, the analyses of organic dyes allowed identifying indigo and gallic acid in more than one sample adding fundamental information about Chinese artists’ techniques in mural paintings, missing from the previous studies. To shed light on the gilding technique, the distribution of the painting materials was achieved by means of synchrotron radiation Fourier transform infrared spectroscopy (SR micro-FTIR) and X-ray fluorescence (SR micro-XRF). The results obtained from the multi-analytical approach enabled us to determine the organic materials both binders and organic colorants used by Chinese artisans, highlighting the high technical level achieved in nineteenth century. The binding media and the organic colorants identified, as well as their distribution, allowed the discussion on the painting technique used by the artists of the Qing dynasty giving information for the first time about the decoration of Chinese civil buildings.
 |
N. Salvadó, S. Butí, M. Cotte, G. Cinque and T. Pradell, "Shades of green in 15th century paintings: combined microanalysis of the materials using synchrotron radiation XRD, FTIR and XRF", Applied Physics A, 111, 47-57 (2013). |
A representative selection of green paintings from fifteenth century Catalonia and the Crown of Aragon are analyzed by a combination of synchrotron radiation microanalytical techniques including FTIR, XRD, and XRF. The green pigments themselves are found to be a mixture of copper acetates/basic copper acetates and basic copper chlorides. Nevertheless, a broader range of green shades were obtained by mixing the green pigment with yellow, white, and blue pigments and applied forming a sequence of micrometric layers. Besides the nature of the pigments themselves, degradation and reaction products, such as carboxylates, formates and oxalates were also identified. Some of the copper based compounds, such as the basic copper chloride, may be either part of the original pigment or a weathering product. The high resolution, high brilliance, and small footprint of synchrotron radiation proved to be essential for the analysis of those submillimetric paint layers made of a large variety of compounds heterogeneous in nature and distribution and present in extremely low concentrations.
 |
A. Lluveras-Tenorio, A. Andreotti, I. Bonaduce, S. Boularand, M. Cotte, J. Roque, M. Colombini and M. Vendrell-Saz, "Mass Spectrometric and Synchrotron Radiation based techniques for the identification and distribution of painting materials in samples from Paints of Josep Maria Sert", Chemistry Central Journal, 6, 45 (2012). |
Establishing the distribution of materials in paintings and that of their degradation products by imaging techniques is fundamental to understand the painting technique and can improve our knowledge on the conservation status of the painting. The combined use of chromatographic-mass spectrometric techniques, such as GC/MS or Py/GC/MS, and the chemical mapping of functional groups by imaging SR FTIR in transmission mode on thin sections and SR XRD line scans will be presented as a suitable approach to have a detailed characterisation of the materials in a paint sample, assuring their localisation in the sample build-up. This analytical approach has been used to study samples from Catalan paintings by Josep Maria Sert y Badía (20th century), a muralist achieving international recognition whose canvases adorned international buildings.
The pigments used by the painter as well as the organic materials used as binders and varnishes could be identified by means of conventional techniques. The distribution of these materials by means of Synchrotron Radiation based techniques allowed to establish the mixtures used by the painter depending on the purpose.
Results show the suitability of the combined use of SR μFTIR and SR μXRD mapping and conventional techniques to unequivocally identify all the materials present in the sample and their localization in the sample build-up. This kind of approach becomes indispensable to solve the challenge of micro heterogeneous samples. The complementary interpretation of the data obtained with all the different techniques allowed the characterization of both organic and inorganic materials in the samples layer by layer as well as to establish the painting techniques used by Sert in the works-of-art under study.
 |
M. Cotte, J. Susini, V. A. Solé, Y. Taniguchi, J. Chillida, E. Checroun and P. Walter, "Applications of synchrotron-based micro-imaging techniques to the chemical analysis of ancient paintings", Journal of Analytical Atomic Spectrometry, 23, 820-828 (2008). |
Ancient paintings are complex materials in terms of chemical analysis because they are usually made of organic/mineral, amorphous/crystallized, major/minor mixtures, evolving with time, and organized in micrometric multi-layered arrangements. In this context, synchrotron micro-imaging techniques offer a powerful analytical platform to reveal the two dimensional atomic, molecular and structural compositions of such complex systems, at a micrometre resolution. The two selected examples illustrate the two main concerns of restorers and conservators: looking backwards, to get insight into ancient artistic practices (in particular through the identification of pigments and binders in Bamiyan Buddhist mural paintings); and looking forward, to preserve works of art as long as possible (through a better understanding of cinnabar blackening in Medieval Spanish paintings). From the analytical chemistry point of view, they also illustrate the relevance of combining micro X-ray fluorescence, micro X-ray absorption spectroscopy, micro X-ray diffraction, and micro-FTIR for the complete analysis of painting cross-sections (binders and pigments).
 |
J. Dik, K. Janssens, G. van der Snickt, L. van der Loeff, K. Rickers and M. Cotte, "Visualization of a lost painting by Vincent van Gogh visualized by synchrotron radiation based X-ray fluorescence elemental mapping", Analytical Chemistry, 80, 6436-6442 (2008). |
Vincent van Gogh (1853−1890), one of the founding fathers of modern painting, is best known for his vivid colors, his vibrant painting style, and his short but highly productive career. His productivity is even higher than generally realized, as many of his known paintings cover a previous composition. This is thought to be the case in one-third of his early period paintings. Van Gogh would often reuse the canvas of an abandoned painting and paint a new or modified composition on top. These hidden paintings offer a unique and intimate insight into the genesis of his works. Yet, current museum-based imaging tools are unable to properly visualize many of these hidden images. We present the first-time use of synchrotron radiation based X-ray fluorescence mapping, applied to visualize a woman’s head hidden under the work Patch of Grassby Van Gogh. We recorded decimeter-scale, X-ray fluorescence intensity maps, reflecting the distribution of specific elements in the paint layers. In doing so we succeeded in visualizing the hidden face with unprecedented detail. In particular, the distribution of Hg and Sb in the red and light tones, respectively, enabled an approximate color reconstruction of the flesh tones. This reconstruction proved to be the missing link for the comparison of the hidden face with Van Gogh’s known paintings. Our approach literally opens up new vistas in the nondestructive study of hidden paint layers, which applies to the oeuvre of Van Gogh in particular and to old master paintings in general.
|
Lluveras, S. Boularand, J. Roqué, M. Cotte, P. Giráldez and M. Vendrell-Saz, "Weathering of gilding decorations investigated by SR: Development and distribution of calcium oxalates in the case of Sant Benet de Bages (Barcelona, Spain)", Applied Physics A, 90, 23-33 (2008). |
In this paper seventeenth century gilded paintings from the crypt of Sant Benet de Bages, a medieval monastery in the Catalonia region of Spain, near Barcelona, have been studied. Cross sections from two different gilded decorations were studied by means of optical microscopy and electron microscopy and EDS to determine the statigraphy and elemental composition, and by means of FTIR coupled to a microscope to determine the binding media associated to each layer. These preliminary results demonstrated that gilded decorations were made by the application of a gold foil on a mordant substrate on a gypsum base, while the mouldings of the vaults seem to be gilded on a bol with a glaze on top of the gold leaf. It is interesting to notice that the first remained unaltered, while the gilded vault mouldings look almost black, due to the darkening of the organic material. To elucidate the causes involved in the darkening of the sample from the vaults a set of synchrotron μXRD and μFTIR experiments have been carried out on these samples at the ESRF (ID18F and ID21, respectively). High brightness and small spot working conditions revealed the development and distribution of calcium oxalates in the binding media, which seem to be responsible for the darkening. Results point out the fact that weddellite (CaC2O4·2H2O) is the phase formed in those layers where organic material has also been identified or at least it would be supposed to be by bibliographic sources and not necessarily those superficial as it would have been suggested due to the similarities with patinas formation.
 |
M. Cotte, E. Welcomme, V. A. Solé, M. Salomé, M. Menu, P. Walter and J. Susini, "Synchrotron-based X-ray spectromicroscopy used for the study of an atypical micrometric pigment in 16th century paintings", Analytical Chemistry, 79, 6988-6994 (2007). |
Grünewald is a famous German painter of the 16th century, whose celebrity is associated with his unique skill in handling colors. This article presents the analysis of materials used to render a metallic aspect in the Isenhein Altarpiece and the Basel's Crucifixion. Such samples are challenging objects for microanalysis due to both chemical and physical complexity. Their study by synchrotron-based X-ray microscopy techniques was made possible thanks to recent developments carried out at the ID21 beam line (European Synchrotron Radiation Facility, ESRF). A submicron X-ray fluorescence probe revealed the main presence of lead, sulfur, antimony, and calcium. The fluorescence-line interferences (in particular K-lines of sulfur with M-lines of lead, and K-lines of calcium with L-lines of antimony) were resolved with the fitting program, PyMCA. 2D-mapping highlighted the presence of micrometer grains of sulfur and antimony into a lead matrix. XANES measurements were performed at both the sulfur K-edge and the antimony L-edge to refine information from an atomic to a molecular level. Beam stability was a key point in this study to selectively probe micrometer pigment grains, dispersed in the lead matrix. They confirm that the grains are made of stibnite (antimony sulfide), a very atypical pigment. Chemical mapping of sulfides is perfectly correlated with antimony mapping and provides a clear visualization of the stibnite pigments, in addition to their identification. Besides its artistic relevancy, this work aims at illustrating developments of synchrotron X-ray microprobe methods for the chemical characterization and observation of complex and micrometer-scale materials.
|
M. Cotte, E. Checroun, J. Susini and P. Walter, "Micro-analytical study of interactions between oil and lead compounds in paintings", Applied Physics a-Materials Science & Processing, 89, 841-848 (2007). |
Oil paintings are complex hybrid materials, made of organic binders associated with inorganic minerals, susceptible to evolving over centuries. In particular, interactions of oil with lead compounds may give rise to the formation of lead soap aggregates, so-called protrusions. This phenomenon is studied here via X-ray and FTIR micro-analysis of an ancient painting dated from 1610. In complement, the synthesis of modern preparations, reconstructed from ancient recipes was assessed. Molecular and atomic images are obtained by combining synchrotron-based FTIR and X-ray fluorescence microscopies. Protrusions are identified in both ancient and modern samples, more particularly, in the ground layer of the paintings, below the colored layer. These observations imply that lead oxide, introduced as a siccative and not as a pigment, may be the element mainly responsible for the protrusions formation, and that this degradation may appear very rapidly on paintings.



