- Home
- News
- Spotlight on Science
- Electrolyte-driven...
Electrolyte-driven nanostructuring improves C-C coupling during CO2 electroreduction
18-11-2019
Electrolyte-driven nanostructuring can be used to synthesise highly-selective Cu catalysts for CO2 electrochemical reduction to valuable multicarbon products. Operando spectroscopy measurements showed that, in addition to the effect of the enhanced roughness, subsurface oxygen, Cu(I) species and adsorbed halides favour C-C coupling.
The electrochemical production of fuels and chemical feedstocks from CO2 and water using electricity derived from renewable energy holds promise as a sustainable process that might help to mitigate some of our current energy and climate challenges. The CO2 electrochemical reduction reaction (CO2RR) is a complicated catalytic process due to the broad range of products that can be obtained at high overpotential. Multicarbon oxygenate and hydrocarbon products (C2+) with high energy density are highly desirable, but severely limited by the slow kinetics of multiple proton and electron transfer steps during C-C coupling. Copper is the only metal able to produce hydrocarbons and alcohols in considerable amounts. However, polycrystalline Cu usually suffers from the need to employ high overpotentials and its low C2+ product selectivity. The formation of C2+ products during CO2RR is very sensitive to the catalyst structure [1-4]. It is desirable, therefore, to develop nanostructured electrocatalysts through rational design that could be capable of efficient generation of multicarbon products from CO2RR.
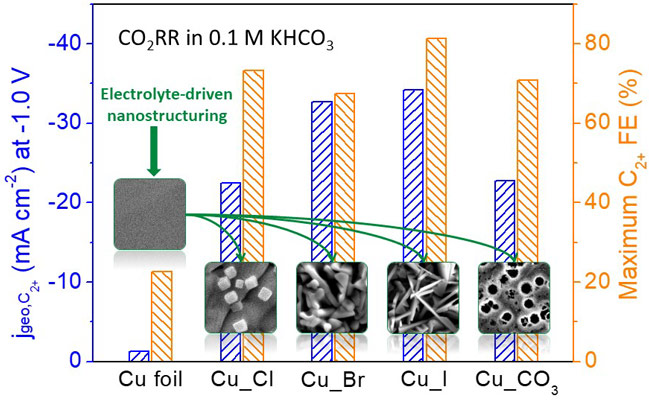
Surface reconstructions are commonly observed in heterogeneous catalysis, with the most active catalysts dynamically adjusting to the electrolyte, applied potential and other reaction conditions [5-7]. Here, an efficient electrochemical strategy has been developed, namely, electrolyte-driven nanostructuring, for the synthesis of high surface area nanostructured Cu catalysts for the selective generation of C2+ products from CO2RR. This approach consists of the electrochemical modification of an electropolished Cu foil by successive oxidation and reduction pre-treatment cycles in different electrolytes. The originally flat surface is transformed into well-defined nanostructures with dimensions and shape that are strongly dependent on the electrolyte anion employed (Figure 1). For instance, chlorine-modifications lead to the growth of Cu nanocubes on the polycrystalline Cu surface, while iodine-modifications produce needle-like Cu structures. Interestingly, the newly-created rough Cu catalysts show a suppressed methane formation but improved CO2RR selectivity towards ethylene and multicarbon alcohols as compared to the flatter pristine Cu foils. The iodine-modified Cu catalyst displays the highest Faradaic Efficiency of ~80% for C2+ products at -0.9 V vs RHE (reversible hydrogen electrode).
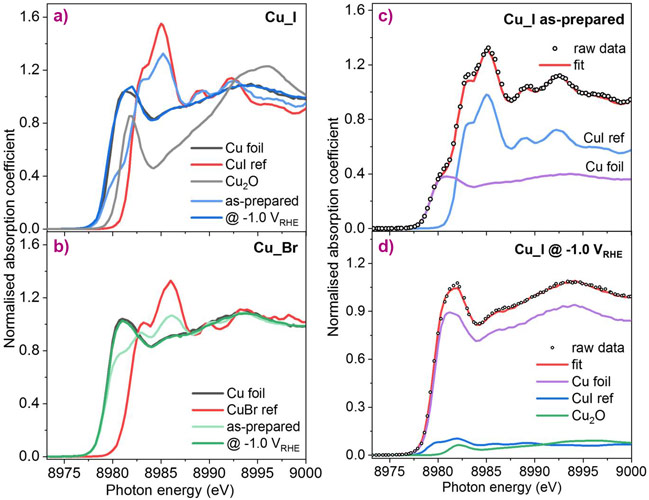
It is well known that the activity and selectivity of CO2RR catalysts strongly depend on the precise control of their structure, such as the content of defects, subsurface oxygen or Cu(I) species, the specific shape/facet of the nanocrystals, or the surface composition and atomic ordering in bimetallic nanostructures. To uncover the reaction mechanism behind the improved CO2RR selectivity of our halide-modified Cu catalysts, operando high energy resolution fluorescence-detected X-ray absorption near edge structure (HERFD-XANES) measurements were conducted at BM20, the Rossendorf Beamline. This technique is very sensitive to the chemical state and coordination environment of Cu and allows the residual Cu(I) species under reaction conditions to be observed. Operando spectra show that some Cu(I) species can still survive in the form of Cu-halide and/or Cu2O at the negative potentials relevant to CO2RR (Figure 2). Of all the halide-modified Cu catalysts, the iodine-modified one has the highest Cu(I) content, which is consistent with the highest Faradaic efficiency for C2+ products ever observed. These important high-resolution synchrotron experiments provided direct evidence of the positive correlation between the presence of Cu(I) species during CO2RR and improved C2+ production. From this, it can be postulated that Cu(I) species are active sites for C-C coupling, but the specific reaction pathways must be further investigated in close collaboration with theorists.
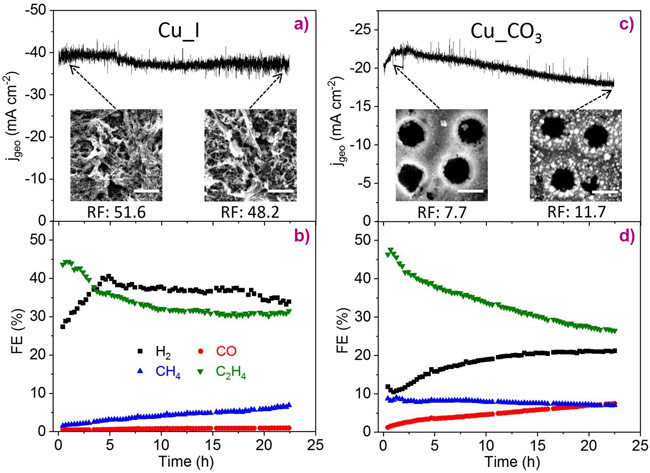
Since Cu(I) species (e.g., Cu2O and CuI) are thermodynamically unstable at the negative potentials employed for CO2RR, it is likely that they become gradually depleted in the course of a long-term reaction. In fact, stability tests show decreased ethylene formation but increased methane production with time (Figure 3), further suggesting the vital role of Cu+ species in the formation of C2+ products. In clear contrast, a carbonate-modified reference Cu catalyst sample with no Cu(I) species present during CO2RR, shows stable methane production but increased CO and H2 formation. The latter trend is attributed to the formation of small nanoparticles with an enhanced number of low-coordinated sites where a strong hydrogen adsorption is favoured.
In combination with other characterisation techniques such as quasi in situ XPS, it can be concluded that the high C2+ selectivity of these nanostructured Cu catalysts can be attributed to the highly roughened surface morphology induced by their synthesis, the presence of subsurface oxygen and Cu(I) species as well as the adsorbed halides. This work provides new insight into the parameters that can be tuned in order to rationally design C2+-selective CO2 electroreduction catalysts.
Principal publication and authors
Selective CO2 electroreduction to ethylene and multicarbon alcohols via electrolyte-driven nanostructuring. D. Gao (a), I. Sinev (a,b), F. Scholten (a,b), R.M. Aran-Ais (a), N.J. Divins (a,b), K. Kvashnina (c,d), J. Timoshenko (a), B. Roldan Cuenya (a), Angew. Chem. Int. Ed. 58, 17047-17053 (2019); doi: 10.1002/anie.201910155.
(a) Department of Interface Science, Fritz Haber Institute of the Max Planck Society, Berlin (Germany)
(b) Department of Physics, Ruhr-University Bochum (Germany)
(c) Rossendorf Beamline at ESRF – The European Synchrotron, Grenoble (France)
(d) Institute of Resource Ecology, Helmholtz Zentrum Dresden-Rossendorf (HZDR), Dresden (Germany)
References
[1] R.M. Aran-Ais et al., Acc. Chem. Res. 51, 2906 (2018).
[2] D. Gao et al., ACS Nano 11, 4825 (2017).
[3] P. Grosse et al., Angew. Chem. Int. Ed. 57, 6192 (2018).
[4] H. Mistry et al., Nat. Commun. 7, 12123 (2016).
[5] D. Gao et al., ACS Catal. 7, 5112 (2017).
[6] D. Gao et al., ACS Catal. 8, 10012 (2018).
[7] D. Gao et al., Nat. Catal. 2, 198 (2019).