- Home
- News
- Spotlight on Science
- Towards a perfect...
Towards a perfect chemical reactor
05-07-2019
A perfect chemical reactor would provide separated, pure products and it would overcome the chemical equilibrium limitations of reversible reactions. Research involving beamline ID22 shows how such a reactor could be made.
The aim of the research described here was to demonstrate a perfect reactor using an approach called chemical looping [1]. The research aims to prove that such a reactor can overcome equilibrium limitations whilst using a reversible reaction of societal importance. Hydrogen production is an important large-scale chemical process that could well underpin any future low-carbon energy economy. It is therefore important to develop new, more efficient approaches. Key to these processes is the water-gas shift (WGS) reaction:
H2O + CO ⇄ H2 + CO2 Reaction 1
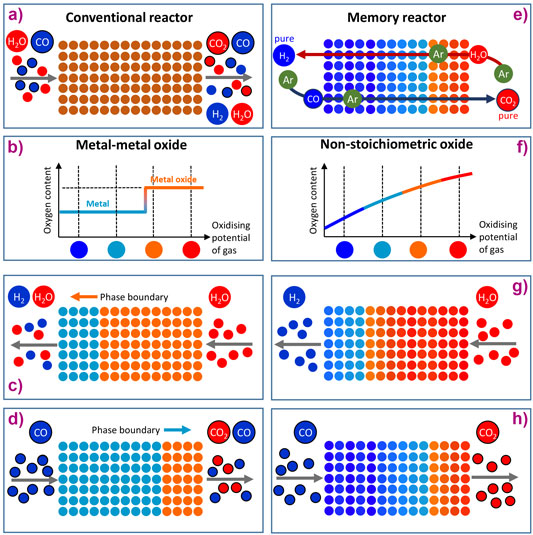
where H2O is reacted with CO to produce H2 and CO2. In a conventional hydrogen production process, the WGS reaction is equilibrium-limited and requires two reactors operating at progressively lower temperatures to achieve high conversion while H2 and CO2 must then be separated (see Figure 1a with its mix of different products).
To perform the WGS reaction in chemical looping (CL) mode, H2O (high oxidising potential) is passed over a solid-phase oxygen carrier material (OCM) that accepts oxygen, splits H2O and produces H2, which is illustrated in Figure 1c. In a second step, CO (low oxidising potential) is passed over the OCM, removing the oxygen and at the same time producing CO2, Figure 1d. The big thermodynamic advantage of this (in theory at least) is that the reactants H2O and CO are not mixed. However, in practice this advantage is not realised because conventional OCMs such as metal/metal oxide systems function by donating and receiving oxygen at one fixed oxygen chemical potential (Figure 1b). Figures 1c and 1d show that a conventional metal/metal oxide OCM cannot convert H2O fully to H2 or CO to CO2.
The idea presented here involves using an oxide of variable non-stoichiometry able to donate and receive oxygen over a range of conditions. The oxide used was La0.6Sr0.4FeO3-δ, a perovskite oxide. Because this is non-stoichiometric (see Figure 1f), it can exist in many difference states of δ and thus better accommodate both half cycles. A video has been produced to aid visualisation of how the material and reactor behave: video link.
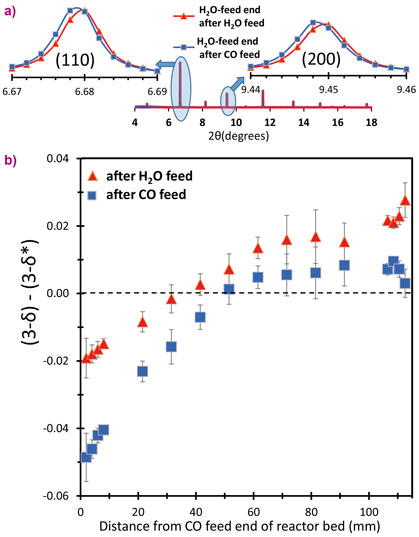
If this reactor operates as designed, it should, on cycling, develop an oxidised low-δ end to the reactor (the H2O-feed end) and a reduced high-δ end to the reactor (the CO feed end). There should be a range of δ values in the bed between these extremes. Beamline ID22 was used to experimentally probe δ in a working reactor (working at 817°C for the chemistry to function and also because the equilibrium constant of the WGS reaction is unity at this temperature). When non-stoichiometric oxides lose oxygen they expand slightly, therefore, an indication of the local value for δ can be obtained from the lattice parameter dependence (from XRD). The advantage of using synchrotron is rapidity: data can be collected much faster than the time constants that govern the reactor (the time between switching from one feed to the other). Very good angular resolution permitted small changes in lattice parameter to be measured, and high energy and intensity X-rays were able to pass through a fully functioning model reactor. An example of the results is shown in Figure 2, demonstrating that oxidising and reducing ends to the bed have been developed.
In summary, the reactor gives much higher conversions than predicted by equilibrium, is 100 times more effective than a conventional approach (using a suitable measure of reactor performance) and produces pure H2 at one end and pure CO2 at the other eliminating the need for complex product separation.
Principal publication and authors
Overcoming chemical equilibrium limitations using a thermodynamically reversible chemical reactor, I.S. Metcalfe (a), B. Ray (a), C. Dejoie (b), W. Hu (a), C. de Leeuwe (a), C. Dueso (a), F.R. García-García (c), C.-M. Mak (a), E.I. Papaioannou (a), C.R. Thompson (a) and J.S.O. Evans, Nature Chemistry 11, 638-643 (2019); doi: 10.1038/s41557-019-0273-2.
(a) School of Engineering, Newcastle University (U.K.)
(b) ESRF
(c) School of Engineering, The University of Edinburgh (U.K.)
(d) Department of Chemistry, Durham University (U.K.)
References
[1] A. Thursfield, A. Murugan, R. Franca, I.S. Metcalfe, Chemical looping and oxygen permeable ceramic membranes for hydrogen production-a review, Energy & Environ. Sci. 5, 7421-7459 (2012).