- Home
- Users & Science
- Find a beamline
- Complex systems and biomedical sciences
- ID17 - Biomedical Beamline
- Bronchography: Lung structure and function studied by K-edge subtraction imaging
Bronchography: Lung structure and function studied by K-edge subtraction imaging
Introduction
Obstructive lung diseases, such as asthma and COPD, cause regional changes in lung structure and function. These diseases are clinically studied by global methods such as spirometry or functional imaging techniques (PET, MRI), where resolution is poor. None of clinically available imaging techniques allows simultaneous high-resolution imaging of both central airway geometry and of the quantitative distribution of regional ventilation, limiting the ability to study structure-function relationships.
The K-edge Subtraction (KES) method was originally developed for human coronary angiography with iodine contrast (Rubenstein et al.1986, Elleaume et al. 2000, Suortti and Thomlinson, 2003) and later extended to lung imaging with stable xenon gas as contrast agent (Giacomini et al. 1998, Bayat et al. 2001). KES imaging is an ideal tool for studying lung function, since it allows simultaneous imaging of central conducting airway dimensions and quantitative measurement of regional ventilation in experimental models of induced airway obstruction (Bayat et al. 2006). Moreover, lung perfusion can be studied simultaneously using iodine in the blood as the contrast agent (Suhonen et al. 2008).
Materials and methods
KES imaging is performed simultaneously with two X-ray beams, above and below the xenon K-edge energy Bayat et. 2001). Subtraction of these two mono-energetic images on a logarithmic scale allows the observation of small anatomic structures carrying the contrast agent, while removing practically all features due to other structures (Figure 1). X-rays from a synchrotron radiation source are required since they allow the selection of monochromatic beams from the full X-ray spectrum while conserving enough intensity for imaging. A CT image provides a quantitative distribution of xenon gas within the airways, and of the regional gas volume (Monfraix et al. 2005). Dynamic imaging during xenon wash-in allows the measurement of regional specific ventilation, i.e. the ventilation normalized to voxel volume (Porra et al. 2004). Animal models (rabbit, rat, mouse) are used to study lung diseases, such as asthma and allergy, and the effects of irritants and medication.
The mono-energetic beams are produced from the continuous synchrotron radiation spectrum by a bent silicon crystal monochromator and the energy difference between the beams is typically 250 eV, beam width is 15cm and height 0.7mm. The beams focus and crossed at the animal position, beyond which they diverge and are recorded by either a liquid nitrogen-cooled high-purity germanium dual-line detector (width 15cm, pixel width 350?m) or a FreLoN camera (pixel size 50?m) (Elleaume et al. 1999).
The ventilator set-up consists of a custom-made apparatus, including a T-tube and electromagnetic valves, which are used to synchronize mechanical ventilation with the image acquisition. During the resting period the animal breaths air, and during imaging a mixture of xenon (Xe 70%) and oxygen (O2 30%). Ventilation is paused in expiration for image acquisition in order to avoid movement artifacts during imaging. Ventilator is combined with Forced Oscillation Technique setup that allows simultaneous studies of global lung function parameters.
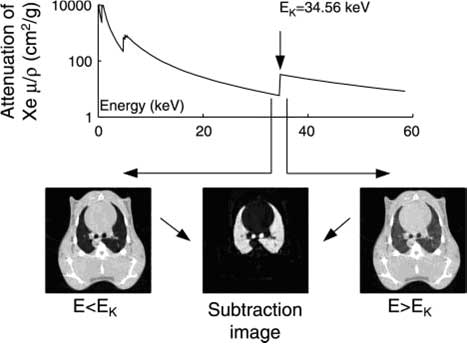
Figure 1: In K-edge subtraction imaging (KES), two simultaneous CT images are acquired using two x-ray beams at two different energies above and below the K-edge of xenon. Absolute quantity of the contrast agent is determined directly on any given point of a lung CT image after subtracting these two images on a logarithmic scale. (Bayat et al. 2006).
Results
KES imaging has been used to study experimental asthma caused by histamine aerosol in healthy rabbits. Histamine provocation causes airway narrowing and heterogeneous changes in the ventilation. There are substantial differences in the kinetics of response in central and peripheral airways to inhaled histamine. Large central airways show slower response to histamine inhalation and were maximally constrict only when small peripheral airways had significantly recovered (Bayat et al. 2006, Bayat et al. 2009).
Experimental asthma studies have been extended to study allergic reactions by using ovalbumine-sensitized rabbit model (Figure 2). Allergic reactions were compared with experimental asthma reactions caused by non-specific drug provocation (Methacholine, Mch). Non-specific provocation (Mch) caused airway narrowing mainly on the large airways, while allergen (ovalbumin) caused heterogeneous changes in the peripheral ventilation (Bayat et al. 2009). The results are highly relevant for critical care medicine.
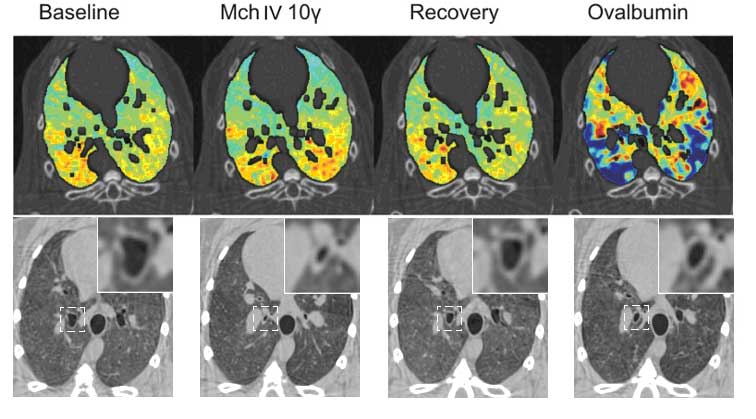
Figure 2. Upper panel: images of specific ventilation in one representative sensitized rabbit at baseline, during methacholine (Mch) infusion, upon recovery and after Ovalbumin allergen provocation; ?: ?g/kg/min. Lower panel: Absorption CT images showing changes in the central airway cross-sectional area at the different experimental stages in one representative animal. Magnifications of the indicated square areas are shown in the right-upper corners. (Bayat et al. 2009).
References
1. Bayat S, Le Duc G, Porra L, Berruyer G, Nemoz C, Monfraix S, Fiedler S, Thomlinson W, Suortti P, Standertskjöld-Nordenstam C-G, Sovijärvi ARA. Quantitative functional lung imaging with synchrotron radiation using inhaled xenon as contrast agent. Phys Med Biol 46: 3287-3299, 2001.
2. Bayat S, Porra L, Suhonen H, Nemoz C, Suortti P, and Sovijarvi AR. Differences in the time course of proximal and distal airway response to inhaled histamine studied by synchrotron radiation CT. J Appl Physiol 100: 1964-1973, 2006.
3. Bayat S, Porra L, Suhonen H, Suortti P and Sovijärvi ARA. Paradoxical conducting airway response and heterogeneous regional ventilation after histamine inhalation in healthy anaesthetized rabbits studied by synchrotron radiation CT. J Appl Physiol 2009; 106: 1949–1958.
4. Bayat S, Strengell S, Porra L, Janosi TZ, Petak F, Suhonen H, Suortti P, Hantos Z, Sovijarvi AR, and Habre W. Methacholine and ovalbumin challenges assessed by forced oscillations and synchrotron lung imaging. Am J Respir Crit Care Med 180: 296-303, 2009.
5. Elleaume H, Charvet AM, Berkvens P, Berruyer G, Brochard T, Dabin Y, Dominguez MC, Draperi A, Fiedler S, Goujon G, Le Duc G, Mattenet M, Nemoz C, Perez M, Renier M, Schulze C, Spanne P, Suortti P, Thomlinson W, Esteve F, Bertrand B, Le Bas JF. Instrumentation of the ESRF medical imaging facility. Nucl Instr and Meth A 428: 513-527, 1999.
6. Elleaume H, Fiedler S, Esteve F, Bertrand B, Charvet AM, Berkvens P, Berruyer G, Brochard T, Le Duc G, Nemoz C, Renier M, Suortti P, Thomlinson W, and Le Bas JF. First human transvenous coronary angiography at the European Synchrotron Radiation Facility. Phys Med Biol 45: 39-43, 2000.
7. Giacomini JC, Gordon H, O'Neil R, Van Kessel A, Cason B, Chapman D, Lavendar W, Gmur N, Menk R, Thomlinson W, Zhong Z, and Rubenstein E. Bronchial imaging in humans using xenon K-edge dichromography. Nucl Instr and Meth in Phys Res A 406: 473-478, 1998.
8. Monfraix S, Bayat L, Porra L, Berruyer G, Nemoz C, Thomlinson W, Suortti P and Sovijärvi ARA. Quantitative measurement of regional lung gas volume by synchrotron radiation computed tomography. Phys Med Biol 50: 1-11, 2005.
9. Porra L, Monfraix S, Berruyer G, Nemoz C, Thomlinson W, Suortti P, Sovijärvi ARA, Bayat S. Effect of tidal volume on distribution of ventilation assessed by synchrotron radiation CT in rabbit. J Appl Physiol 96: 1899–1908, 2004.
10. Rubenstein E, Hofstadter R, Zeman HD, Thompson AC, Otis JN, Brown GS, Giacomini JC, Gordon HJ, Kernoff RS, Harrison DC, Thomlinson W. Transvenous coronary angiography in humans using synchrotron radiation. Proc Natl Acad Sci USA 83(24): 9724-9728, 1986.
11. Suhonen H, Porra L, Bayat S, Sovijärvi AR, Suortti P. Simultaneous in vivo synchrotron radiation computed tomography of regional ventilation and blood volume in rabbit lung using combined K-edge and temporal subtraction. Phys Med Biol. 2008 Feb 7;53(3):775-91.
12. Suortti P, Thomlinson W. Medical applications of synchrotron radiation – topical review. Phys Med Biol 48: R1-R35, 2003.



