- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2011
- Structure of materials
- The missing link: surface freezing meets self-assembly
The missing link: surface freezing meets self-assembly
The formation of organic monolayers is of interest both for fundamental science and for applications ranging from biosensors to organic photovoltaic devices. At one end of the organic monolayer spectrum are self assembled monolayers (SAMs), like gold-supported alkyl-thiols and silica-supported alkyl-silanes, where the solution prepared monolayer formation and order are dominated by the strong covalent bond between the head group and substrate while the weaker chain-chain van der Waals (vdW) interactions are less important. At the other end of the spectrum is surface freezing, where a solid monolayer of n-alkane molecules forms at the free surface of their own melt over a temperature range of a few degrees above the bulk melting point. Here the lateral vdW interaction between molecules dictates the frozen monolayer’s structure, while the very weak interaction of the molecules with the “substrate” (the vapour) plays only a minor role. The present study is the first to explore the intermediate regime, where both chain-chain and substrate-head group interactions are of equal strength and importance in determining the lateral structure of the monolayer. This study provides, therefore, a hitherto-missing link between self-assembly and surface freezing.
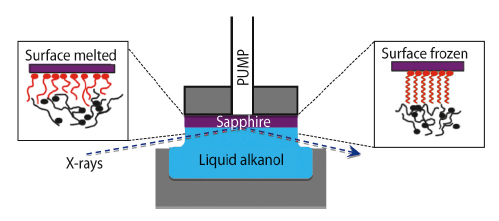
Using X-ray reflectivity at the high energy microfocus beamline ID15A, we have investigated the structure of long-chain alcohol monolayers at the interface of a highly polished sapphire (0001) surface while in contact with either a molten bath or a saturated vapour of the same long-chain alcohol. In Figure 48 the experimental configuration is shown where the sapphire surface is in contact with the bulk molten alcohol. X-rays of 71 keV were focused to <10 mm using compound refractive optics lenses and then reflected off the liquid/solid interface. The reflectivity recorded as a function of the incidence angle a expressed as qz = (4π/λ)sinα, where λ = 0.175 Å is the X-ray wavelength. Using a model for the surface-normal electron density, the monolayer’s structure can be extracted from the measured reflectivity.
 |
|
Fig. 48: Schematic of the X-ray scattering geometry at the liquid/solid interface (centre). The fit-derived molecular models are shown in the disordered state (left) and in the surface frozen state (right). |
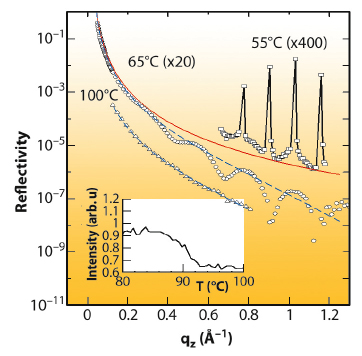
Figure 49 shows the reflectivity at the C18OH/sapphire interface at several temperatures along with the Fresnel reflectivity, RF, the theoretical reflectivity of an ideally flat and smooth interface. At 62°C, above the bulk freezing point, Tf = 59.8°C, the reflectivity exhibits well-defined modulations whose qz period corresponds to a single layer of upright molecules. By 100°C, the modulations have nearly vanished suggesting that the monolayer has melted. The melting of the monolayer is clearly visible between 87 and 92°C, as demonstrated by the gradual intensity change that occurs in this interval (see inset to Figure 49). The surface melting transition occurs at a relative transition temperature ΔT = Tb–Tf, which is about 30°C higher than the bulk melting transition. This ΔT is about an order of magnitude larger than that observed for the surface freezing of alkanes [1] and alcohols [2] at the melt/vapour interface. The reflectivity at 55°C, a temperature below Tf, exhibits Bragg peaks characteristic of a crystalline bilayer phase.
 |
|
Fig. 49: Absolute X-ray reflectivity versus qz at the sapphire/C18OH interface at 55, 65 and 100°C corresponding to the crystalline (1 |
Additional reflectivity measurements show that the monolayer remains intact with the same structure and density after removal from the liquid. Complementary grazing incidence diffraction measurements show that the monolayer is ordered commensurately with the underlying sapphire lattice. With increasing temperature, this air terminated “self-assembled monolayer” melts. The monolayer thickness decreases from 25 to 20 Å with a decrease in the number of hydrogen bonded alcohol groups.
Our results provide insight into the link between surface freezing and self-assembled monolayers. The thickness change upon melting and the temperature range of the surface frozen layer are consistent with a simple free energy model that only includes the OH-sapphire adsorption energy, and the elastic penalty associated with stretching the chains. On the basis of this model, we expect that surface freezing is a generic property of all self-assembled monolayers. For self-assembled monolayers, the temperature range of surface freezing will depend on the energetics of the surface bond, the entropy change upon melting, and the availability of surface bonding sites.
Principal publication and authors
B.M. Ocko (a), H. Hlaing (a), P.N. Jepsen (a), S. Kewalramani (a), A. Tkachenko (b), D. Pontoni (c), H. Reichert (c) and M. Deutsch (d), Phys. Rev. Lett. 106, 137801 (2011).
(a) Condensed Matter & Materials Science Department, Brookhaven National Laboratory, Upton NY (USA)
(b) Center for Functional Nanomaterials, Brookhaven National Laboratory, Upton NY (USA)
(c) ESRF
(d) Physics Department and Institute of Nanotechnology, Bar-Ilan University, Ramat-Gan (Israel)
References
[1] X.Z. Wu et al., Phys. Rev. Lett. 70, 958 (1993).
[2] M. Deutsch et al., Europhys. Lett. 30, 283 (1995).



