- Home
- News
- General News
- New technique films...
New technique films the birth of molecules with unprecedented time resolution
21-02-2013
For decades, scientists have been studying the reagents and the products of chemical reactions. However, due to a lack of tools fast enough to catch the action, they’ve not been able to observe directly the movements and play of atoms during the making and breaking of bonds involved in the first moments of a chemical reaction. Now, scientists have developed a technique using time slicing that enables the birth of molecules and the associated rearrangement of solvent molecules to be filmed by taking snapshots every 10 ps. The study was published on 27 February 2013 in the Journal of the American Chemical Society.
Share
The international research team was led by Michael Wulff from the European Synchrotron Radiation Facility (ESRF) in Grenoble and Hyotcherl Ihee from Korea’s Institute for Basic Science and the Korea Advanced Institute of Science and Technology (KAIST).
The first moments of a chemical reaction
Molecules are usually born in high energy states which are accompanied by large amplitude vibrations in the bonds between the atoms. In solution, a newly formed molecule cools down by transferring energy to the surrounding solvent molecules. This process takes place in just a few picoseconds - a few trillionths of a second - and has so far never been directly visualised. Solvent plays an important role in solution-phase chemical reactions by serving as an energy source to activate the reaction as well as a heat bath to stabilise the products. As a result, the properties of the solvent significantly affect the energy landscape, rates and pathways of a reaction in solution. How solute and solvent molecules interact and the effect of this interaction on the outcome of chemical reactions has been a topic of intense research in the field of reaction dynamics for several decades. It is still challenging experimentally to probe the bonds that are formed during a chemical reaction, especially the changes between solute and solvent accompanying the structural change of reacting solute molecules. The challenge for these experiments is to develop new tools that have both sufficient time resolution and structural sensitivity.
Unprecedented time resolution
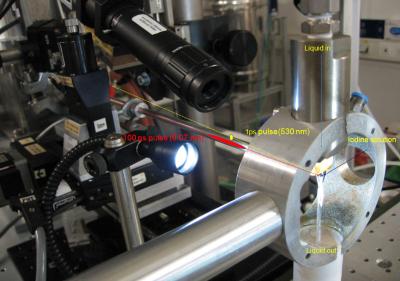
The novel technique of time slicing developed by the researchers consists in using a very short 1 picosecond laser pulse, a hundred times shorter than the X-ray pulse. The laser pulse dissociates the molecule into two atoms that are trapped in the solvent cage. Then a delayed X-ray pulse probes their reassociation at that time delay. The delay is controlled to a precision of 1 ps. The association process is probed in10 ps time steps and the data treated with a new deconvolution algorithm to sharpen the data.
By combining this technique with time-resolved X-ray scattering, the team was able to reach an unprecedented time resolution of 10 ps. Coupled with structural sensitivity offered by the very high pulse intensity from a quasi monochromatic undulator (U17) and a very efficient detector (FReLoN), the scientists were able to measure the dynamics of atom dissociation and reassociation and the vibrational relaxation of I2 in two different solvents.
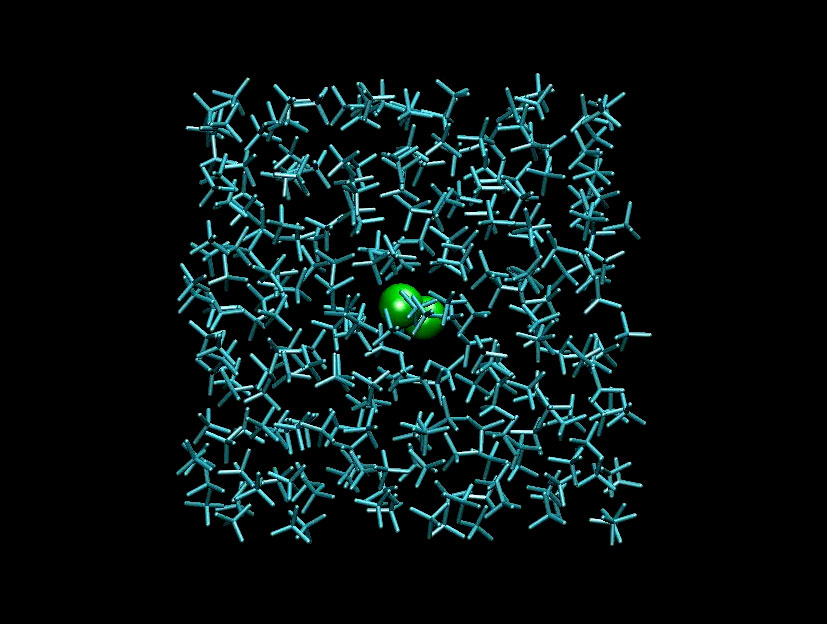
The team found that the I-I bond distance increases from 2.65 Å to a maximum of about 4 Å before the iodine atoms draw back together. The distance differs depending on the solvent: it is greater in cyclohexane than in carbon tetrachloride, which has a higher molecular weight.
A film of the vibrating atoms inside the solvent cage was finally made by stitching together 30 snapshots taken at delays starting from 0 ps to 300 ps. Click on the photo below to view the animation.
 |
|
|
The experiments were carried out at the ESRF’s ID09B beamline which specialises in the study of structural dynamics. The beamline is equipped with a high speed chopper that is used as a fast mechanical shutter to select the flash produced by a single bunch out of the high frequency train of flashes produced by the storage ring.
“The unique filling patterns of the storage ring, which have been developed since the outset of the institute in collaboration with the accelerator division, and specialised chopper technology, have provided us with the tools available to achieve these unique results”, says M. Wulff co-author of the publication. “We hope now to take the experiments further and observe the quantum nature of chemical bond formation, most probably in collaboration with the European X-FEL”.
References
Filming the birth of molecules and accompanying solvent rearrangement, J.H. Lee, M. Wulff, S. Bratos, J. Petersen, L. Guerin, J.-C. Leicknam, M. Cammarata, Q. Kong, J. Kim, K.B. Møller and H. Ihee, J. Am. Chem. Soc. 135, 3255–3261 (2013).
Top image: Researchers filmed the dissociation of the iodine molecule and the subsequent reassociation and vibrational relaxation of the molecule.