- Home
- News
- General News
- Growing catalysts
Growing catalysts
08-12-2006
PRESS RELEASE - Porous materials are involved in many chemical reactions that affect our daily lives. Despite their wide use, there is little knowledge about them. Scientists from the Netherlands, United Kingdom and the ESRF have just shed new light on how these materials organise themselves when they are created. Their experiments at the DUBBLE beamline at ESRF combined three different techniques in real time, with the aim of viewing a full picture of the process. This new information could help improve their synthesis in the future.
Share
“Zeolites” might be an unknown word to many non-scientists, but their influence is everywhere around us: when we wash our clothes, drive a car or walk in the streets. They are used in many processes, such as the production of petrol, detergents or concrete. They are inorganic porous material with a highly regular structure of channels and pores that allow some molecules to pass through, and cause others to be either excluded, or broken down. In nature, they are made of volcanic rock, but industry has been synthesising them for many years. In industry they are formed from a gel and only become (catalysts) or porous solids when templates are used to direct the formation of a structure. If organic bases (chemical compounds which can neutralise an acid) are added to the reaction, new structures can be formed, but the way this happens is not well understood. A deeper knowledge of this process would enable better catalysts to be made.
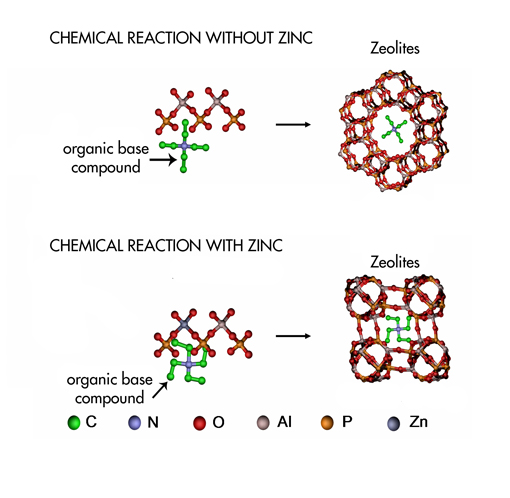 |
The growth of a zeolite. When an organic base is added to the gel, it acts as a template for the construction of the zeolite (upper row). However, when zinc is added into the gel, the organic base takes a different shape, leading to a different construction of the zeolite. Credits: Andrew Beale. |
In order to get new insight on this process, the team of researchers from The Netherlands, United Kingdom and the ESRF monitored the synthesis of zeolites with organic bases in real time. They added zinc to the original gel because it promotes the formation of zeolites at low temperatures. They realised that this element influenced the template of the zeolite and the crystallisation process . The results suggest that molecular organisation of the zeolites occurs before crystallisation, therefore, before the formation of zeolite crystals.
The time-resolved experiments at the beamline BM26A took place on a specially developed setup, and combined three different techniques, namely X-ray absorption spectroscopy and small- and wide-angle diffraction. They complemented these with additional data using Raman spectroscopy. “We could look at each aspect of the crystallisation process for the first time ever”, explains Andrew Beale, one of the researchers, from Utrecht University (The Netherlands).
The new results may not have an immediate repercussion among industrial zeolite manufacturers, but they provide a new vision on these materials for the academic community. The outcome of this research was published in two papers in the Journal of American Chemical Society and has been recently reported in Nature. “These results are highly relevant to the debate on the mechanism of zeolite formation”, asserts Rutger A. Van Santen, a scientist from the Schuit Institute of Catalysis (The Netherlands), in the Nature article.
References
Beale, A. M. et al. J. Am. Chem. Soc. 128, 12386–12387 (2006).
O’Brien, M. G., et al. J. Am. Chem. Soc. 128, 11744–11745 (2006).
Van Santen, R. A., Nature, Vol 444, 46 - 47 (02 Nov 2006).
For further information, please contact Montserrat Capellas, ESRF Press Officer, tel. +33 476 88 26 63.



