- Home
- News
- General News
- Filming an Ultra-fast...
Filming an Ultra-fast Biological Reaction Essential to Life
23-06-2003
A team of scientists from the USA in collaboration with staff at the European Synchrotron Radiation Facility1 (Schotte et al) have managed to film a protein at work in unprecedented detail. The protein is the oxygen-storing molecule myoglobin, which plays a central role in the production of energy in muscles. The motion of the protein was recorded using ultra-short flashes of X-rays from the synchrotron. The new insight in the functionality of myoglobin has led to a deeper understanding of the molecular processes associated with respiration. An article on this subject was published on Friday 20 June in "Science" under the title Watching a Protein as it Functions with 150-ps Time-Resolved X-ray Crystallography.
Share
Every time we contract a muscle, myoglobin releases oxygen, which is used by all mammals in the production of energy. Muscle cells use myoglobin as a peak-load buffer when blood cannot supply oxygen fast enough, for example when the circulation is blocked during muscle contraction. The oxygen molecule is initially confined in a cavity called the heme-pocket, where it is chemically bound to an iron atom.
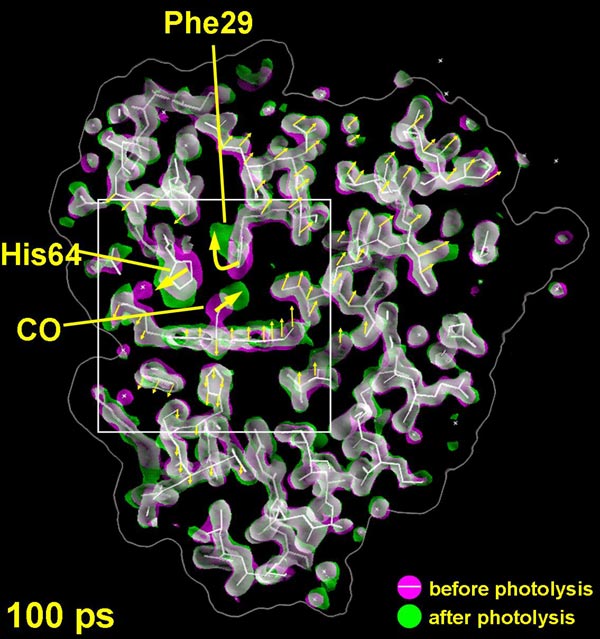
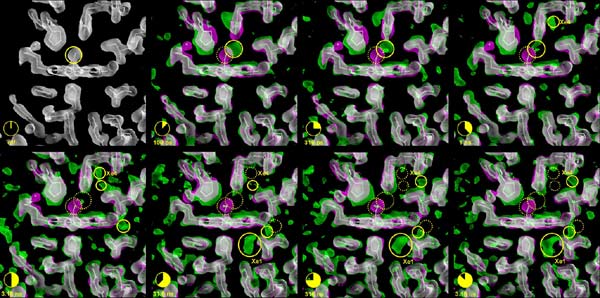
The three-dimensional pictures taken at the ESRF resolve positions of all the 1432 atoms in the protein, and pinpoint how the carbon monoxide (CO) molecule - used here as a replacement for oxygen (O2) for technical reasons - literally finds its way out of the very dense atomic structure near the iron atom. The scientists have discovered that the CO molecule does not move out smoothly; in fact it spends most of its time captured in 5 tiny cavities inside the protein. In the first cavity near the iron atom, the CO molecule makes an extremely brief visit lasting only 100 picoseconds2, i.e. a tenth of a billionth of a second. Iron would naturally try to rebind CO, but nearby molecules block the CO from going back to the iron. The film has shown that the motion between the 5 cavities is very fast. The CO molecule reaches the fifth cavity after 30 nanoseconds and then it disappears into the solvent surrounding the protein. The interesting thing is that eventually another CO molecule, released from a myoglobin molecule nearby, will diffuse back towards the iron, most likely through another route. The iron accepts the incoming CO due to the fact that the structure of the protein has changed to allow for the rebinding.
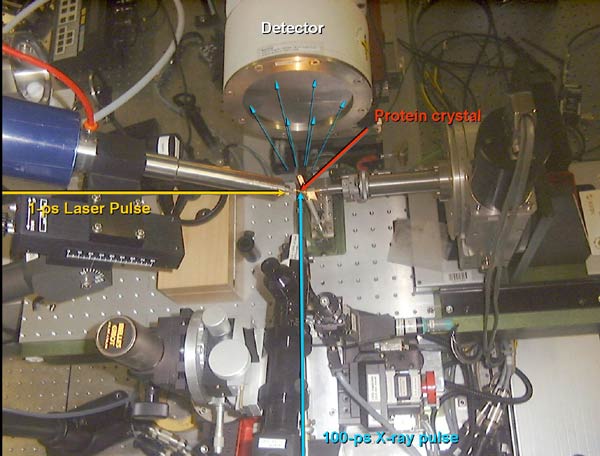
Watching myoglobin as it functions is more complicated than it may seem. First of all, the scientists have to control the start of the experiment extremely precisely. The experiment begins by the injection of a flash of laser light to perturb the molecules and release the CO molecule inside the protein. Very shortly afterwards, they expose the protein crystal to an intense flash of X-rays. The X-rays are scattered by the protein into diffraction3 pictures that are later analysed by computers. The duration of an X-ray flash from the synchrotron is as short as 100 picoseconds and that is in principle the shortest time duration that can be investigated at the ESRF.
About the "scenario" for the filming
The live filming of the protein took place in ID09, one of the 40 beamlines at the ESRF. A beamline is a laboratory for X-ray studies. The X-ray radiation enters the experimental cabin and "shoots" a sample, in this case, a crystallised protein. This beamline, called White Beam Station, is a beamline designed for time-resolved experiments in macromolecular crystallography and liquids, and also for high pressure research. Its unique feature is the focused white beam, which can be used for time-resolved diffraction on macromolecules.
Pictures of this experiment can be found below.
If you need a higher resolution of images or the film, contact Montserrat Capellas, ESRF press officer, tel. +33 476 88 26 63 (press@esrf.fr)
1. The European Synchrotron Radiation Facility (ESRF) is constituted as an international facility with 17 participating countries to operate, maintain and develop a synchrotron radiation source and associated instruments. It operates the most powerful third generation synchrotron radiation source in Europe (www.esrf.fr).
How the synchrotron works
Electrons emitted by an electron gun are first accelerated in a linear accelerator (linac) and then transmitted to a circular accelerator (booster synchrotron) where they are accelerated to reach an energy level of 6 billion electron-volts (6 GeV). These high-energy electrons are then injected into a large storage ring -844 metres in circumference- where they circulate in a vacuum environment, at a constant energy, for many hours. The synchrotron beams emitted by the electrons are directed towards the "beamlines" which surround the storage ring in the experimental hall. Each beamline is designed for use with a specific technique or for a specific type of research. Experiments run throughout day and night.
2. Note that the ratio between 100 ps to 1 second is equivalent to the ratio between 1 sec and 317 years.
3. What is diffraction?
When a crystal is illuminated by a beam of X-rays, it scatters the light. Each atom in the crystal becomes the source of a weak amplitude wave propagating in all directions, and these waves interfere among themselves. As a result, most of the scattering is cancelled, only the strongest scattering remains. These diffracted X-rays fill photographic film with spots that help describe the molecular structure of the crystal.
Images from the Experiment
The structural change measured 100 ps after the CO molecule has left its binding-site next to the iron atom. The change in the electron density is shown in green (gain) and magenta (loss). The initial state of the protein is shown in white.
Migration of the CO after photolysis. The sites that are currently occupied by the CO are marked by solid yellow circles. Sites from which the CO has departed are depicted by a dotted yellow circle (click on the image to view at higher resolution).
The experimental setup in the beamline.