- Home
- News
- Spotlight on Science
- Oxygen "breaths”...
Oxygen "breaths” in Earth’s subduction zones
13-09-2022
Scientists using beamline BM30 have exploited arsenic as an oxygen probe in rocks of the former subduction zone of Himalaya, to reveal O2 release at depths of 100 km during subduction. X-ray absorption spectroscopy data suggest that these so-called oxygen “breaths” may control the transfer of volatile elements from the deep Earth to the surface, shaping our planet’s evolution.
Subduction zones are the most active places of matter recycling on Earth. These fascinating “redox factories” scavenge or release water, oxygen, hydrogen, sulfur and carbon, depending on the element’s oxidation state. When, why, and how much subduction zones “breathe” in and out volatile elements remains poorly understood. This is partially due to the lack of in-situ tracers to record the evolution of redox conditions during different subduction stages.
A team of scientists attempted to fill this knowledge gap by studying the redox state of the trace element arsenic in serpentinite rocks of the Tso Morari massif in north-west Himalaya, which was a subduction zone about 40-50 million years ago, before the mountain rose. These rocks were exhumed from a depth of 100 km during orogenesis, and bear a precious witness of deep subduction phenomena [1].
The researchers carried out X-ray absorption spectroscopy at beamline BM30 (FAME), which enabled in-situ quantification of the chemical and redox state of arsenic trapped in the rocks and minerals. They discovered a large range of arsenic redox states, spanning from –3 (arsenides, i.e., reduced As bound to metals such as nickel or iron, NiAs) to +3 (arsenites, i.e., moderately oxidised As bound to oxygen atoms, AsIIIO3), and +5 (arsenates, i.e., most oxidised arsenic bound to oxygen, AsVO4).
The combination of these findings, complementary laboratory analytical methods [2], thermodynamic modelling of arsenic chemical speciation [3] and the geodynamic history of these rocks [1] resulted in the proposal of a novel model to follow the pathway of arsenic in the subduction zone and to use the element as an oxygen probe (Figure 1).
Click to enlarge
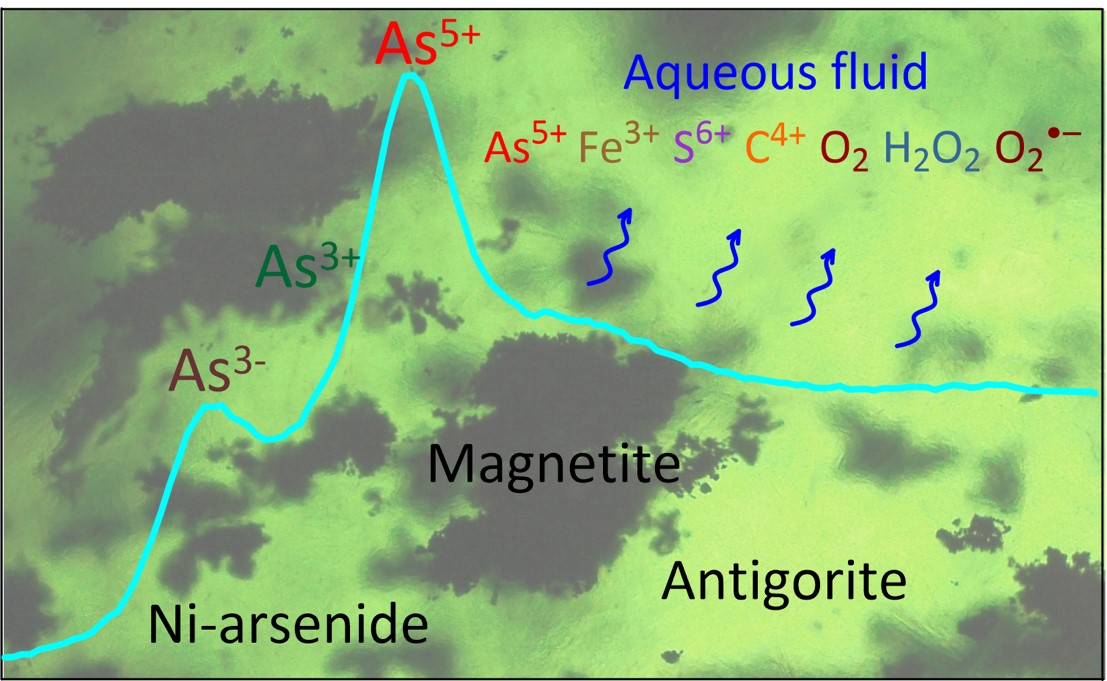
Fig. 1: Schematic picture showing the approach used and the phenomena of oxidising fluid release. The X-ray absorption spectrum (blue curve) provides direct information on the arsenic redox state contained in the major minerals of the serpentinite rock (imaged as the background). The released fluid (undulating blue arrows) carries oxygen and its radicals and major chemical elements in their highest oxidation states. © Gleb Pokrovski.
The presence of arsenides demonstrates that the formation of serpentinite during the early stages of subduction, by the interaction of water with olivine (the major mineral in the Earth’s mantle), massively consumes oxygen. At more advanced subduction stages at greater depths and temperatures, the partial breakdown of serpentinite back to olivine releases highly oxidising aqueous fluids, accompanied by arsenite and arsenate (Figure 2).
Click to enlarge
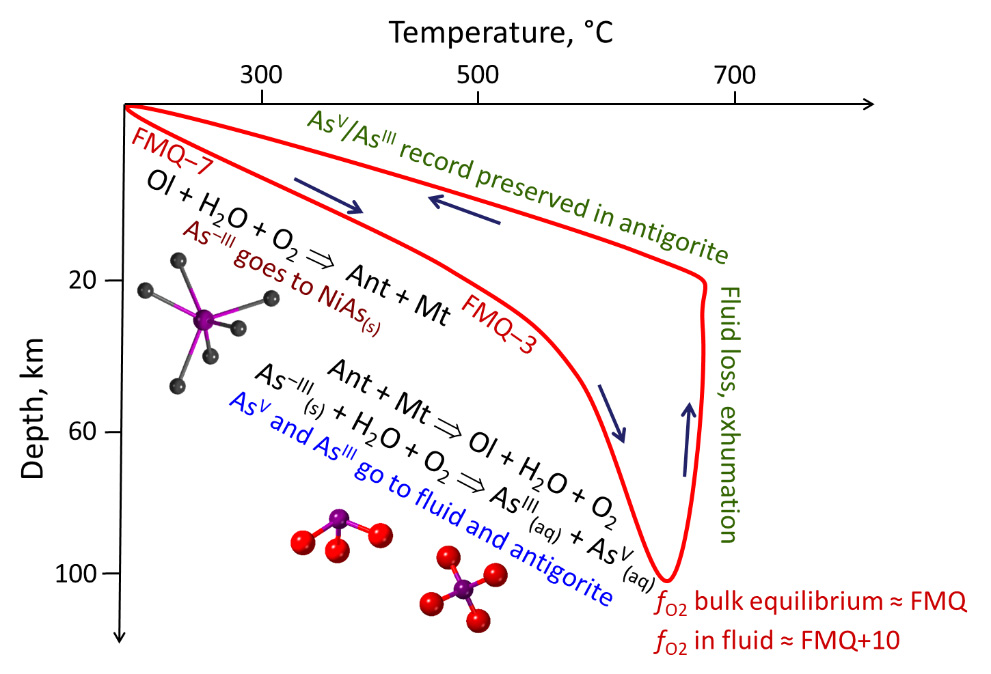
Fig. 2: Arsenic redox transformation reactions along the temperature vs. depth subduction path of the Tso Morari metamorphic rocks [1]. Arrows show the directions of the subduction and subsequent exhumation. Ant = antigorite, Mt = magnetite, Ol = olivine. FMQ = oxygen fugacity reference of the conventional fayalite-magnetite-quartz mineral buffer. Main As species are pictured in ball-and-stick mode (As=violet, O=red, Ni=grey). © Gleb Pokrovski.
Through the measured ratios of arsenic oxidation state in serpentinite, the redox parameters were quantified in the fluid during the different subduction steps. These magnitudes are commonly described by geoscientists as O2 fugacity or apparent partial pressure. The O2 partial pressure, present in these fluids at advanced subduction stages, corresponds to millibar levels at 600-700°C and 100 km depth. Such O2 pressures are about 10 orders of magnitude greater than commonly believed when assuming complete thermodynamic equilibrium between fluid and rock.
Arsenic redox state thus offers a unique route of the focused out-of-equilibrium generation of extremely oxidising fluids – a phenomenon that cannot be captured by traditional petrological approaches. The exact oxygen speciation in these fluids requires further studies; it is possible that molecular oxygen is accompanied by other O-bearing reactive species like O2•− or H2O2, procuring these fluids an exceptional chemical reactivity.
This study opens new avenues for tracing the dynamic phenomena of fluid-rock interactions, often occurring out of equilibrium in subduction zones at depth. These findings will call for future in-situ synchrotron-based studies of fugitive fluids under extreme conditions, inaccessible so far. Overall, this research contributes to our understanding of the fundamental links between the deep Earth and its surface.
Principal publication and authors
Redox dynamics of subduction revealed by arsenic in serpentinite, G.S Pokrovski (a), C. Sanchez-Valle (b), S. Guillot (c), A.Y. Borisova (a), M. Muñoz (d), A.-L. Auzende (c), O. Proux (e), J. Roux (f), J.-L. Hazemann (g), D. Testemale (g), Y.V. Shvarov (h), Geochem. Perspect. Lett. 22, 36-41 (2022); https://doi.org/10.7185/geochemlet.2225
(a) Géosciences Environnement Toulouse, CNRS-Université Toulouse III (France)
(b) Institute of Mineralogy, University of Münster (Germany)
(c) ISTerre, CNRS-Université Grenoble Alpes (France)
(d) Géosciences Montpellier, Université de Montpellier (France)
(e) Observatoire des Sciences de l'Univers de Grenoble, CNRS-Université Grenoble Alpes, Saint Martin d’Hères (France)
(f) Institut de Physique du Globe, CNRS, Paris (France)
(g) Institut Néel, ESRF, Grenoble (France)
(h) Lomonosov Moscow State University, Moscow (Russia)
References
[1] S. Guillot et al., Tectonophysics 451, 225-241 (2008).
[2] A.Y. Borisova et al., Front. Earth Sci. 9, 640464 (2021).
[3] E. Perfetti et al., Geochim. Cosmochimica Acta 72, 713-731 (2008).
|
About the beamline: BM30 (FAME) BM30 FAME, the French X-ray absorption spectroscopy (XAS) beamline, covers a wide variety of scientific fields (materials science, biophysics, chemistry) but focuses mainly on Earth sciences, where the speciation of trace elements in heterogeneous and/or complex samples can be studied. For these reasons, FAME’s optical elements have been designed to maximise the photon flux on the sample and to optimise beamline stability and reduce non-statistical noise, in order to permit XAS measurements down to 10 s of ppms concentrations. The beamline also offers several sample environments: a low temperature helium cryostat for measurements on redox sensitive and diluted elements, and high-pressure and -temperature autoclaves for in-situ hydrothermal and catalysis studies. The SSHADE/FAME open database (https://www.sshade.eu/db/fame) gathers spectra of standards and characteristic samples, enriched with complete metadata. |