- Home
- News
- Spotlight on Science
- Towards a deeper...
Towards a deeper understanding of tick-borne encephalitis virus
30-08-2022
ESRF beamlines were used to determine the crystal structure of a previously elusive intermediate in the infectivity-activation process of tick-borne encephalitis virus, an important human-pathogenic flavivirus closely related to a range of mosquito-transmitted viruses of global importance.
Every year, more than 10,000 people in parts of Europe and Asia are infected by tick-borne encephalitis (TBE) virus, usually transmitted by the bites of infected ticks, causing a serious disease of the central nervous system. However, infections and small outbreaks in humans can also occur after the consumption of raw milk or cheese, primarily from tick-infected goats.
Recently, one such outbreak of alimentary infection was documented in Ain, in eastern France, where the virus had never been detected previously [1], corroborating its potential of geographical expansion to previously unaffected regions as observed in other parts of Europe. TBE virus has a cousin (Powassan virus) that is also encephalitogenic and occurs in North America and the far east of Russia. Importantly, these viruses form a larger group (flaviviruses) with other closely related pathogens that are transmitted by mosquitoes.
The related human diseases include yellow fever, dengue, Zika, West Nile and Japanese encephalitis. Although there are successful vaccines against some of these pathologies, specific antiviral drugs have not yet become available. The common property of vector-transmission of flaviviruses makes them prone to becoming an increasing threat because of global warming and the spread of viral vectors, especially by mosquitoes, to new and yet more temperate regions.
All flaviviruses share a similar genome and particle structure, with the basic processes of virus replication including virion assembly, maturation and release being closely related [2]. Decades of intense studies have provided crucial insight into the structural reorganisation of the flavivirus particle required for fusing the viral membrane with a cellular membrane for entry [3].
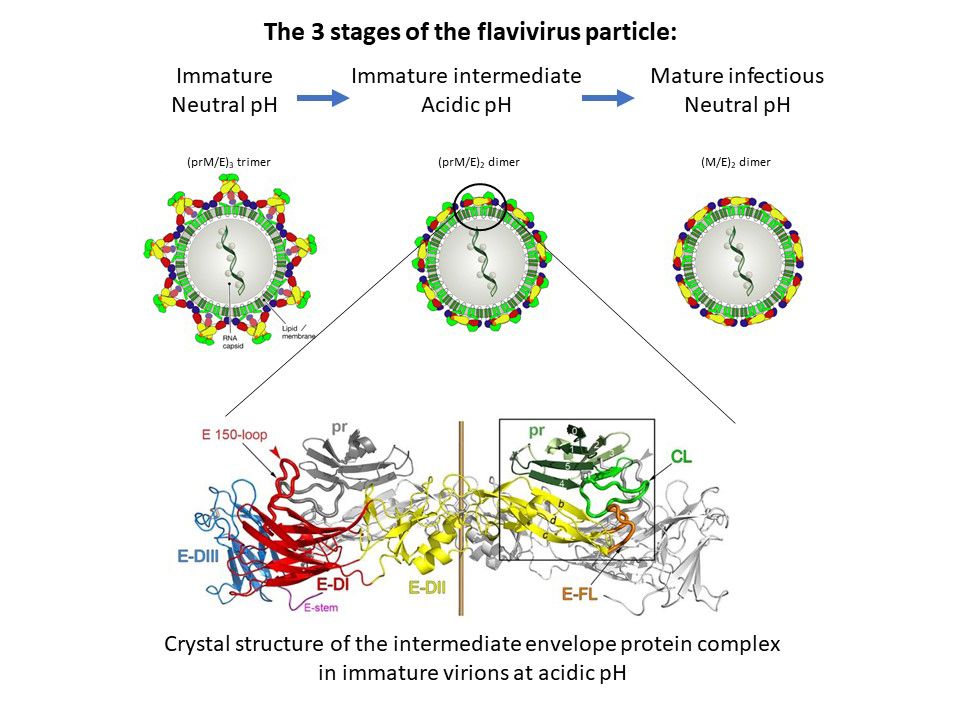
This fusion step is induced by the acidic pH of the endosomes, following uptake of the particle by the target cell. Yet understanding at the molecular level how this essential viral function is controlled and activated during virus assembly and particle release has remained elusive. These processes include oligomeric reorganisations of the two envelope proteins E and prM as well as a proteolytic activation cleavage of prM into pr and M (Figure 1, top panels).
Click to enlarge
Fig. 1: Maturation process of flavivirus envelope proteins. Top: Immature virions containing trimeric spikes of E-prM heterodimers are assembled at neutral pH in the endoplasmic reticulum and transported through exocytosis. The acidic pH in late Golgi compartments induces an oligomeric rearrangement of E and prM as well as cleavage of prM into pr and M, forming smooth-surfaced intermediate immature particles. Upon release from cells and encountering neutral pH, pr falls off to generate fusogenic mature infectious virions (modified from F. Rey et al., EMBO Rep. 2018). Bottom: Side view of the crystal structure of the TBE virus (pr-E)2 complex at acidic pH.
Using beamlines ID23-1 and ID29 at the ESRF, the authors determined the crystal structure of a key intermediate form of the TBE virus envelope glycoproteins that elucidated molecular details of the missing link in our understanding of the processes leading to fusogenic and infectious flavivirus particles (Figure 1, lower panel).
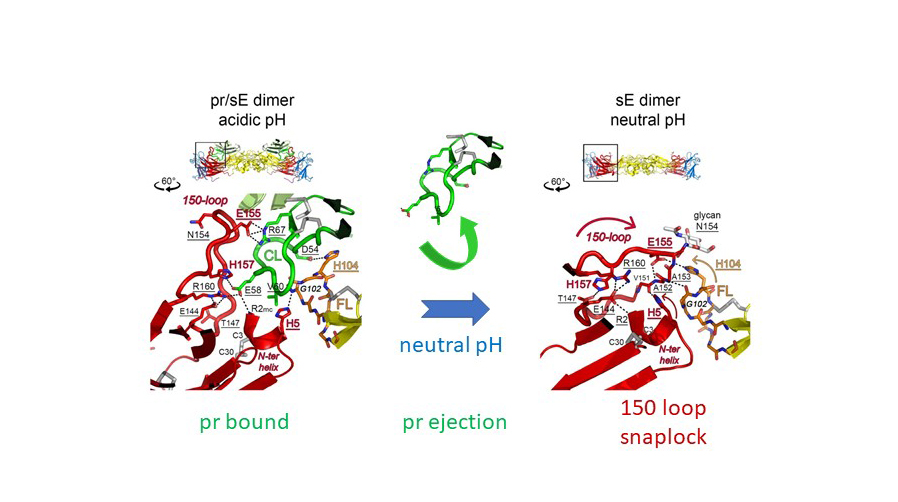
Specifically, they identified a structural element in the viral fusion protein (the 150 loop) that acts as a pH-dependent snap lock and controls the structural and oligomeric rearrangements during the pathways of viral assembly and maturation (Figure 2).
Click to enlarge
Fig. 2: Snap-lock mechanism of the 150-loop in E during virus maturation. At acidic pH (in the trans-Golgi network) the 150-loop opens up to create a positively charged cavity that accommodates pr in immature particles. At neutral pH (upon release of virus particles from infected cells) pr is ejected from the E dimer by the 150-loop, which closes as a hinged-lid to protect the fusion loop (FL) of E. During virus entry, when encountering the acidic pH of endosomes, this snap lock opens again and the exposed fusion loop can initiate membrane fusion.
This new data identifies a new and important site of vulnerability common to all flaviviruses that can become a target for substances inhibiting virus replication, thus providing a lead towards the development of specific antiviral drugs.
Principal publication and authors
Evolution and activation mechanism of the flavivirus class II membrane-fusion machinery, M.-C. Vaney (a), M. Dellarole (a), S. Duquerroy (a), I. Medits (b), G. Tsouchnikas (b), A. Rouvinski (a), P. England (a), K. Stiasny (b), F.X. Heinz (b), F.A. Rey (a), Nat. Commun. 13(1), 3718 (2022); https://doi.org/10.1038/s41467-022-31111-y.
(a) Institut Pasteur, Paris (France)
(b) Center for Virology, Medical University of Vienna (Austria)
References
[1] G. Gonzalez et al., Front. Microbiol. 13, 863725 (2022).
[2] F.A. Rey et al., EMBO Rep. 19, 206 (2018).
[3] F.A. Rey et al., Curr. Opin. Virol. 24, 132 (2017).
|
About the beamlines: ID23-1 and ID29
ID29 is currently being upgraded to EBSL8, a new beamline dedicated to serial macromolecular crystallography experiments. The new beamline will deliver a photon flux density up to 1016 ph/s/µm2 in a 0.5 x 0.5 µm2 at the sample position. Single diffraction images will be collected from micro-crystals at room temperature in a short exposure time, opening new perspectives for time-resolved studies. |