- Home
- News
- Spotlight on Science
- Determination of...
Determination of the kinetic constants of chemical reactions in the millisecond timescale by coupled XAS and UV/Vis spectroscopy
22-03-2019
The kinetic constants of fast bimolecular chemical reactions in solution have been determined for the first time by means of coupled energy-dispersive XAS and UV/Vis spectroscopy. In the near future, structural characterisation of the short-lived reaction intermediates formed during the reactions will be possible from an analysis of the XANES spectra.
An important challenge in modern chemistry is to uncover the structural evolution of the species involved in fast chemical reactions, with time scales ranging from seconds to milliseconds and below. Due to the extreme complexity of this task, it is important to develop new experimental strategies to study the reactivity of such intermediates.
Time-resolved energy-dispersive X-ray absorption spectroscopy (EDXAS) and UV-Vis spectroscopy were combined to investigate the oxidation reactions of organic substrates by a selected non-heme iron activated species. Pseudo first-order kinetic constants related to fast bimolecular reactions in solution were obtained by the time-resolved XAS technique. While XAS allows insight into the local structure and electronic configuration of selected atoms, UV-Vis spectroscopy enables an unambiguous identity assignment of interchanging reaction intermediates: together, the two techniques are complementary instruments for the study of chemical processes.
The research was focused on sulfide and alcohol oxidation by the oxo-complex [N4Py•FeIVO]2+, deriving from the prototypical non-heme iron complex [N4Py•FeII]2+ (N4Py=N,N-bis(2-pyridylmethyl)-N-bis(2-pyridyl)methylamine). The selection of [N4Py•FeIVO]2+ as the oxidising agent was guided by the increasing interest in the use of non-heme iron complexes as catalysts for the oxidation of organic compounds, mainly due to their inexpensive and environmentally friendly nature.
The coupled XAS/UV-Vis approach was employed at beamline ID24 to follow the oxidation of benzyl alcohol to benzaldehyde and the oxidation of a series of thioanisoles, differently substituted in the para position of the aromatic ring, to corresponding methylphenyl sulfoxides. The oxidant [N4Py•FeIVO]2+ was prepared in situ, under experimental conditions already investigated via the parallel XAS/UV-Vis technique in a pioneering study [1]. All reactions were carried out under pseudo-first-order conditions with the concentration of the given arylsulfide or alcoholic species largely exceeding that of [N4Py•FeIVO]2+. In particular, the rate of the iron oxidation state evolution during each reaction was determined by following the relative Fe K-edge energy shift in the time-resolved XAS spectra. The values for the kinetic constants obtained by XAS were in very good agreement with those obtained by means of the concomitant UV-Vis detection.
Figure 1a shows the evolution of the Fe K-edge normalised time-resolved EDXAS spectra relative to the oxidation of PhCH2OH by [N4Py•FeIVO]2+ at selected times from reaction start. The XANES spectra show a shift of the Fe K edge position towards lower energy values, in accordance with the reduction of FeIV to FeII throughout the process. Figure 1b highlights the variation of ΔE0 during the reaction, where ΔE0 is the difference between the K-edge energy position of the XANES spectra at time t and the edge position of the first XANES spectrum at t=0 s, whose experimental decay was fitted with the following equation (blue curve):
where k is the reaction kinetic constant, with a value of 0.11 ± 0.05 s-1.
Figure 1b shows the UV-Vis spectrophotometric data recorded simultaneously on the same reaction mixture. The absorbance variation at 690 nm is due to the decrease in concentration of [N4Py•FeIVO]2+, while the concentration increase of the [N4Py•FeII]2+ species determines the contemporary absorbance increase at 513 nm. The first-order kinetic plots (red and blue curves) provided kinetic constants identical to those determined from the EDXAS spectra.
Figure 2 shows the time evolution of the Fe K-edge EDXAS spectra of the reactions between a) MeOPhSMe, b) PhSMe and c) CNPhSMe with [N4Py•FeIV(O)]2+. In all cases the kinetic constants determined via EDXAS were in good agreement with the k values measured through UV-Vis and with literature data.
The XAS technique, provided quantitative values of the kinetic constants of fast (in the millisecond to second timescales) bimolecular chemical reactions in solution. These results reveal XAS spectroscopy as an innovative tool for the study of fast complex chemical processes involving the oxidation of a metal centre, where the use of other experimental techniques would be impossible. Moreover, the application of multivariate analysis to the time-evolving XAS spectra for a given reaction may allow one to recover the XANES spectra of the short-lived reaction intermediates. The quantitative analysis of these spectra will provide invaluable mechanistic insights into the structures of all of the species formed in the reaction.
Principal publication and authors
Coupled X-ray absorption/UV–vis monitoring of fast oxidation reactions Involving a nonheme iron–oxo complex, G. Capocasa (a), F. Sessa (a), F. Tavani (a), M. Monte (b), G. Olivo (a), S. Pascarelli (b), O. Lanzalunga (a), S. Di Stefano (a), P. D’Angelo (a), J. Am. Chem. Soc. 141, 2299-2304 (2019); doi: 10.1021/jacs.8b08687.
(a) Department of Chemistry, University “La Sapienza”, Rome, (Italy).
(b) ESRF
References
[1] G. Olivo et al., J. Phys. Chem. Lett. 8, 2958-2963 (2017).
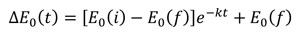
![Oxidation of PhCH2OH by [N4Py•FeIV(O)]2+ followed using the coupled XAS/UV-Vis technique](/files/live/sites/www/files/news/spotlight/2019/spotlight336/spotlight336-fig1sm.jpg)
![Time evolution of the Fe K-edge EDXAS spectra of the reactions between a) p-CH3OC6H4SCH3 (MeOPhSMe), b) PhSCH3 (PhMe) and c) p-CNC6H4SCH3 (CNPhSMe) with [N4Py•FeIV(O)]2+](/files/live/sites/www/files/news/spotlight/2019/spotlight336/spotlight336-fig2sm.jpg)



