- Home
- News
- Spotlight on Science
- Charge-density mapping...
Charge-density mapping reveals hydrogen bond symmetrisation in natrochalcite under high pressure
20-08-2025
High-pressure single-crystal X-ray diffraction was used to map charge-density changes during hydrogen bond symmetrisation in the mineral natrochalcite. Microfocused, highly stable X-rays at beamline ID27 enabled precise electron-density analysis at high pressure. The findings provide new insight into hydrogen bond behaviour in minerals and hydrogen-rich superconductors under extreme conditions.
Share
Upon compression, hydrous minerals and other inorganic and molecular solids containing hydrogen bonds (HBs) undergo a characteristic transformation involving the hydrogen atom. At pressures corresponding roughly to lower-mantle depths, the distinction between the donor and acceptor in the HB disappears, and the bond becomes symmetric and very strong. However, the microscopic nature of this phenomenon is not well understood, due to a lack of systematic studies and the limitations of experimental methods that can detect such subtle changes.
Understanding HB symmetrisation is important, as the formation of strong symmetric HBs in hydrous minerals facilitates the transport of water to deeper parts of Earth’s mantle [1] and also significantly influences the superconductivity of hydrogen sulphide, which currently has the highest known critical temperature (Tc) [2]. In this study, a charge-density analysis based on high-pressure single-crystal diffraction data was used to investigate the mechanism of HB symmetrisation in the mineral natrochalcite [NaCu₂(SO₄)₂·H₃O₂] under compression.
Analysing electron-density distributions refined against X-ray data at high pressure is challenging. The use of diamond anvil cells (DACs) typically results in reduced resolution, lower completeness, and limited data quality. These limitations can be mitigated by employing very bright X-ray sources with short-wavelength radiation, that provide high resolution and better completeness at high pressure.
For this work, extremely high-quality single-crystal X-ray diffraction data were collected at high pressure on beamline ID27 using a microfocused and highly stable X-ray beam, enabling refinement of a quantitative multipole model for detailed electron-density analysis. This approach allowed the detection of subtle electron-density changes and provided direct insight into the mechanism of HB symmetrisation.
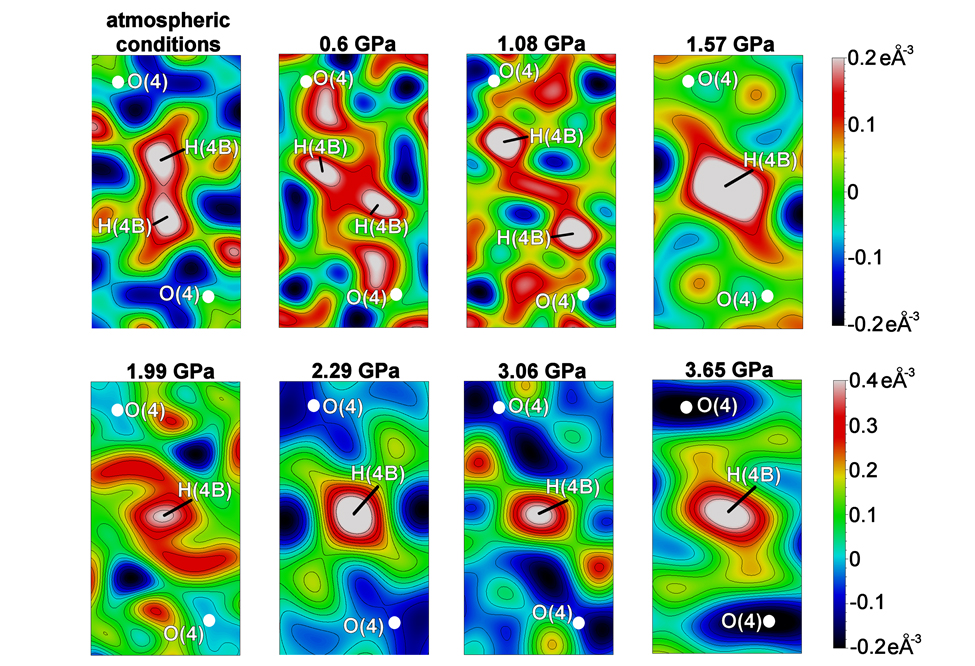
Residual Fourier electron-density maps revealed that, at room temperature and ambient pressure, the hydrogen atom is disordered over two positions in the natrochalcite structure (phase I). At approximately 1.57 GPa, a single positive electron density peak appears between the oxygen atoms (phase II), indicating that hydrogen-atom ordering and HB symmetrisation occur between 1.08 and 1.57 GPa. At ambient pressure, the disordered hydrogen atoms are nearly collinear with the O(4) atom. As pressure increases (to 0.6 and 1.08 GPa), the electron-density peaks move apart, indicating a non-linear configuration. The disordered hydrogen atoms also shift off-centre towards the oxygen atoms, forming a very short O–H covalent bond and a longer H···O hydrogen bond at 1.08 GPa (Figure 1).
Click figure to enlarge
Fig. 1: Residual Fourier electron-density maps showing pressure-induced changes in electron density associated with the disordered hydrogen atom (phase I; atmospheric – 1.08 GPa) and the ordered hydrogen atom (phase II; 1.57 GPa – 3.65 GPa) hydrogen atom. The scales on the right indicate the maximum and minimum residual-density values for the maps in each row (e Å–3).
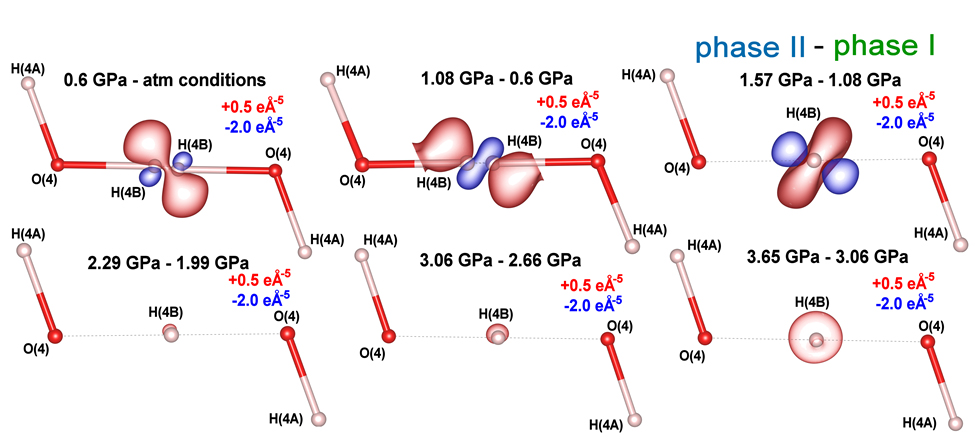
Maps of the negative Laplacian of the electron density for hydrogen H(4B) show regions where electron density increases with pressure (red iso-contours) and regions of depletion (blue iso-contours), with pressure increments as labelled in Figure 2. A significant concentration of electron density away from the O(4)···O(4) axis is observed as pressure increases from ambient conditions to 0.6 GPa and 1.08 GPa. This indicates that the shift of disordered hydrogen atoms into a nonlinear, off-centre arrangement is driven by a redistribution of electron density – specifically, a flow away from the hydrogen-bond centre towards the oxygen atoms at 1.08 GPa. Between 1.08 GPa and 1.57 GPa, the electron density at the bond centre increases markedly; the previously depleted central region becomes enriched, signalling the formation of a symmetric bond (Figure 2). Importantly, the evolution of the relevant order parameter remains continuous, consistent with a second-order transition.
Click figure to enlarge
Fig. 2: 2D maps of the differences in negative Laplacian of the electron density for the hydrogen atom, showing changes in charge concentration and depletion between the pressure points indicated at the top of each panel. Red contours denote regions of charge concentration (iso-contours at +0.5 e Å–5), and blue contours denote regions of charge depletion (iso-contours at −2.0 e Å–5). The maps reveal a complex reorganisation of electron density in the hydrogen bond as a function of pressure.
Additional 3D visualisations of the negative Laplacian of electron density (is-contours at + 0.5 e Å-5 and - 2.0 e Å-5) for successive pressure intervals (0.6 GPa – atmospheric, 1.08 GPa – 0.6 GPa, and 1.57 GPa – 1.08 GPa) illustrate the concentration of electron density in reddish regions and depletion in bluish regions. These images reveal a distinct reversal in electron-density polarisation between 1.08 GPa and 1.57 GPa. This 3D perspective corresponds to the upper row of Figure 2.
These findings show that HB symmetrisation in natrochalcite is unequivocally a second-order phase transition. The process yields a less compressible structure due to the formation of a symmetric HB with unusually strong covalent character. Such changes have important consequences for the physical properties of minerals under mantle-like conditions and for high-pressure hydrogen-rich superconductors. Hydrogen-bond symmetrisation is preceded by continuous rearrangements of disordered hydrogen atoms, including their counterintuitive off-centre movement towards oxygen atoms under compression. This work captures the detailed reorganisation of electron density within a strong HB as a function of pressure.
Principal publication
Symmetrization of Strong Hydrogen Bond under High Pressure in Bihydroxide-Ion-Containing NaCu2(SO4)2·H3O2 Revealed by Experimental Charge Density, Single-Crystal Electron Diffraction, and Neutron Diffraction Studies, P. Rejnhardt et al., J. Am. Chem. Soc. 147 (30), 26830–26843 (2025); https://doi.org/10.1021/jacs.5c08310
References
[1] S. Zhu et al., J. Am. Chem. Soc. 139 (35), 12129–12132 (2017).
[2] I. Errea et al., Nature 532 (7597), 81–84 (2016).
| About the beamline: ID27 |
|
The recently upgraded beamline ID27 addresses some of the most exciting and challenging questions at extremely high pressures and temperatures, such as exploring the conditions deep inside planets, searching for room-temperature superconductivity, and synthesizing new super-hard materials. The beamline supports a range of sample environments, including the double-sided laser-heating system, the Paris-Edinburgh press, the nano-stage and the high-pressure helium cryostat. With higher photon flux, smaller beam sizes (300 × 300 nm2), and improved detector systems compared to its predecessor, ID27 enables a new class of ultra-high-pressure experiments (P> 4 Matm), time-resolved experiments with millisecond-scale resolution, 2D micro-fluorescence mapping, and in-situ X-ray imaging. |