- Home
- News
- General News
- Scientists create...
Scientists create a plastic-eater enzyme that breaks down plastic
16-04-2020
A French team from the Toulouse Biotechnology Institute and the green-chemistry company Carbios has engineered an enzyme that breaks down plastic in hours, giving hope to better recycling treatments. The researchers came to the ESRF to solve the structure of the new enzyme. Their results are published in Nature.
Share
We have all seen the pictures of underwater animals threatened by surrounding plastic in the middle of the ocean. This is testament to a reality where plastic recycling processes are less than optimal today. Estimates show that out of the 359 million tons of plastics produced annually worldwide, 150–200 million tons accumulate in landfills or in the natural environment. Today, French scientists from the Toulouse Biotechnology Institute (TBI, Université de Toulouse, CNRS, INRAE, INSA) and the start-up Carbios have engineered an enzyme that can convert 90% of poly(ethylene terephthalate) (PET) back to its starting materials. And that, within hours.
Poly(ethylene terephthalate) (PET) is the most abundant polyester plastic, with almost 70 million tons manufactured annually worldwide for use in manufacture bottles, polyester clothing fibers, food containers, and various thermoformed packaging and components. The main recycling process for PET, via thermomechanical means, results in a loss of mechanical properties. As an example, only about 30% of the plastic that goes into bottles gets turned into new plastic. This new plastic is often of lower quality, so it is used in carpets or textiles that will ultimately end up in landfills anyway.
In 2012, Japanese scientists found an enzyme that could break down PET plastics, within a microbe present in compost heaps. The enzyme, called leaf-branch compost cutinase (LCC), cuts bonds between two building blocks in PET plastics, terephthalate, and ethylene glycol. Unfortunately, the reaction reaches only a low depolymerization yield at 65°C due to thermal denaturation.
 |
|
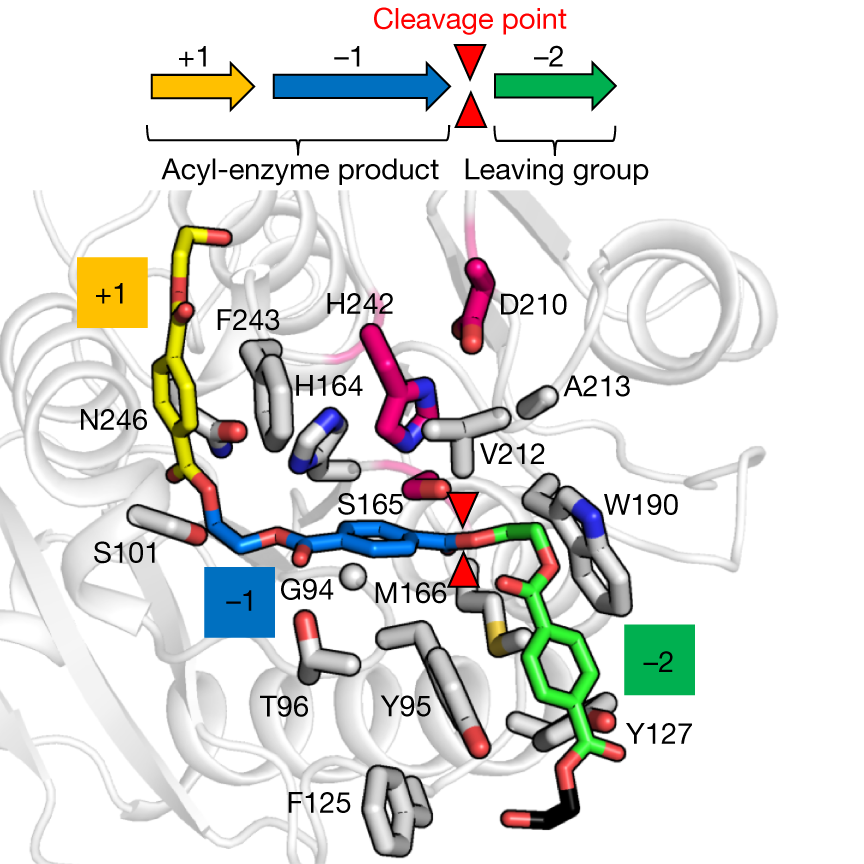
Structural model of 2-HE(MHET)3 (coloured stick model) docked in wild-type LCC (grey ribbon). The putative substrate-binding site of LCC can be subdivided into three subsites (−2, −1, +1), each in contact with the MHET units numbered relative to the scissile ester bond (red triangles). Amino acids in the first contact shell of LCC are shown as grey rods. Catalytic residues are in magenta. Credits: Tournier, V., et al., Nature 580, 216–219 (2020). |
“We screened several cutinases formerly reported to break down PET, including the LCC, which turned out to be the best, after being forgotten for many years”, explains Sophie Duquesne, researcher at the TBI. The team reengineered the enzyme and switched out the amino acids for activity-improving and heat-stabilizing ones. To that end, thanks to the access to the crystallization facility provided by the Structural Biophysics Team of the Institute of Pharmacology and Structural Biology (IPBS, Toulouse), the team collected data from hundreds of crystals on ID30B beamline at the ESRF and at the synchrotron ALBA and subsequently, TBI researchers solved the structure of the new enzyme. The novel enzyme can biologically depolymerize all PET plastic waste in an extremely efficient way by increasing the degradation yield of PET waste to 90% in 10 hours, a significant upswing from the initial degradation yield of 1% after several weeks. “This experiment shows how synchrotron sources, and in particular the ESRF, can do its bit to contribute to tangible new technology that will lead us to a cleaner environment in the years to come”, says Gordon Leonard, head of structural biology at the ESRF.
 |
|
ESRF scientists carry out an experiment on ID30B. Credits: S. Candé. |
“Carbios’ recycling process, the first of its kind, initiates a real transition to a circular economy and can better prevent plastic pollution from harming our oceans and planet”, explains Alain Marty, professor at the University of Toulouse and Carbios’ Chief Scientific Officer, as well as corresponding author of the paper. “This innovative technology also paves the way for recycling PET fibers, another major challenge in guaranteeing a clean and protected environment for future generations”, he concludes.
Reference:
Tournier, V., et al., Nature 580, 216–219 (2020). https://doi.org/10.1038/s41586-020-2149-4
Text by Montserrat Capellas Espuny.



