- Home
- News
- General News
- Research gives clues...
Research gives clues to CO2 trapping underground
08-08-2018
CO2 is an environmentally important gas that plays a crucial role in climate change. It is a compound that is also present in the depth of the Earth but very little information about it is available. What happens to CO2 in the Earth’s mantle? Could it be eventually hosted underground? A new publication in Nature Communications unveils some key findings.
Share
Carbon dioxide is a widespread simple molecule in the Universe. In spite of its simplicity, it has a very complex phase diagram, forming both amorphous and crystalline phases above the pressure of 40 GPa. In the depths of the Earth, CO2 does not appear as we know it in everyday life. Instead of being a gas consisting of molecules, it has a polymeric solid form that structurally resembles quartz (a main mineral of sand) due to the pressure it sustains, which is a million times bigger than that at the surface of the Earth.
Researchers have been long studying what happens to carbonates at high temperature and high pressure, the same conditions as deep inside the Earth. Until now, the majority of experiments had shown that CO2 decomposes, with the formation of diamond and oxygen. These studies were all focused on CO2 at the upper mantle, with a 70 GPa of pressure and 1800-2800 Kelvin of temperature.
A team of scientists from the European Laboratory for Non-linear Spectroscopy (LENS), the University of Florence, the National Research Council of Italy, the University of Vienna and the ESRF came to the high-pressure beamline ID27 to study, using x-ray diffraction and Raman scattering (the latter performed in the facilities of LENS), what happens to CO2 at the depth of 2000 to 2400 kilometres, i.e. at the boundary between the silicate minerals of the lower mantle and the metallic core.
“One of the added value of our team is the fact that we all have different backgrounds: from chemists, to mineralogists and the physicists of the ESRF. This means that we complement each other and, together, we try to get a full picture of what happens to CO2 from our different points of view”, explains Dziubek, corresponding author of the study.
In order to achieve these conditions, they used a diamond anvil cell and submitted the sample to 2400 degrees Celsius (2700K) and 120 GPa of pressure, which is almost double than previous research. "It was a very complex setup, in particular the laser heating with a 10 micron infrared laser at pressures above 100 GPa was very challenging", explains Mohamed Mezouar, scientist in charge of ID27. Thinking that they would come up with similar results to existing literature, they were in for a surprise: CO2 is, in fact, stable in a crystalline form and does not dissociate like previously believed.
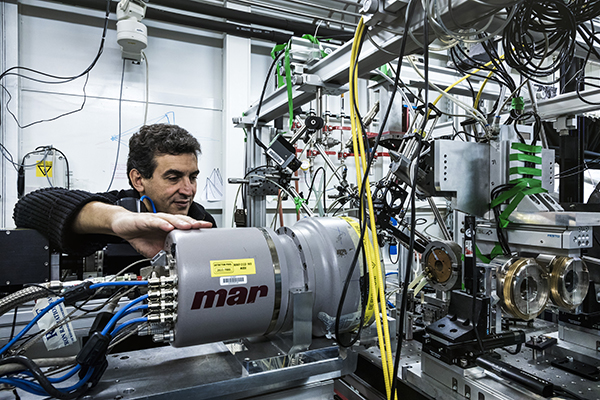 |
|
Mohamed Mezouar, scientist in charge of ID27, on the beamline. Credits: S. Candé. |
“Our results indicate that the crystalline extended form of carbon dioxide is stable in the thermodynamic conditions of the deep lower mantle and therefore could be helpful to understand the distribution and transport of carbon in the depths of our planet. It could even open doors to the possibility of trapping CO2 underground, if it stays there or just in its polymeric form”, explains Kamil Dziubek.
CO2 sequestration in geological formations is one of the potential solutions for mitigating the climate changes associated with the greenhouse effect. It is important, however, to investigate the fate of carbon dioxide in deep geosphere and to recognize the form in which it can be stored within the host rock. If the neat polymeric CO2 stays stable in the deep mantle, it can represent a long-term storage of carbon.
Therefore, the next step of the team is to mimic the real conditions not only in the terms of thermodynamics but also geochemistry, and study in detail stability and reactivity of the CO2 in presence of silicates, carbonates and other minerals, which are known to exist in the deepest parts of the Earth’s mantle.
Text by Montserrat Capellas Espuny
Reference:
Dziubek KF et al, Nature Communications, 8 August 2018. DOI: 10.1038/s41467-018-05593-8
Top image: The Earth, with all its layers.



