- Home
- News
- General News
- How does disorder...
How does disorder cause high heat capacity in glasses? Actually, it doesn’t.
15-01-2014
X-rays have helped solve a long-standing puzzle of solid state physics: why glasses have a higher heat capacity than crystals although they are the same material in different states. Glass is disordered while crystal is ordered, however both have the same number of atoms, the same atomic mass and the same chemical bonding. They should have the same heat capacity. However, at lower temperatures, glass has a much higher heat capacity than crystals.
The question has kept physicists scratching their heads for the last fifty years or so. In the late 1950’s, scientists found that at temperatures of around 10 K (-263°C) glasses need much more heat to warm up than crystals. Soon after, the higher heat capacity of glasses was attributed to a distinct feature in the spectrum of atomic vibrations: At frequencies of about 1 THz, glasses exhibit additional states above the level expected from acoustic waves. This launched an impressive amount of studies that failed to converge into a unified answer as to how disorder causes these anomalies.
Heat capacity can be defined as the amount of thermal energy required to warm the sample by 1°C.
With the whole physics community believing that disorder was the cause of the difference in heat capacity, it took surprising intuition, if not sheer luck, for the scientists working at the European Synchrotron (ESRF) to discover that in fact the answer lies in the density of the materials rather than in disorder.
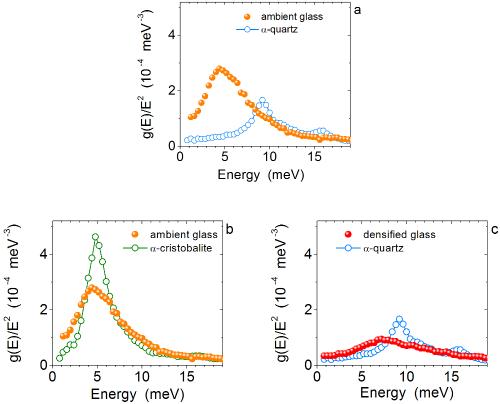
At low energies, typical glass has larger reduced density of states (DOS) than typical crystal (a), and this leads to a higher heat capacity. However, this is not caused by disorder, but by a different density: for glasses and crystals with matched densities, the reduced DOS is nearly the same (b, c), which leads to the same heat capacity.
Using the novel X-ray techniques developed on the ESRF’s Nuclear Resonance (ID18) and Inelastic Scattering (ID28) beamlines, the international team of scientists working under the direction of Aleksandr Chumakov found that disorder does not change the heat capacity.
They decided to study silicon dioxide, the most common mineral on the Earth’s surface, which has several glassy and crystalline polymorphs with different structures and densities. By chance, it turned out that this material has one glass and crystal pair with the same low density and, in addition, another glass and crystal pair with the same high density.
The ESRF scientists involved in the research in front of the ID18 experimental hutch. Credit: ESRF/C. Argoud
They measured spectra of atomic vibrations and found that all (both glassy and crystalline) samples have the same number of the additional states. In some samples, however, these states are located at lower energy and, therefore, provide a higher heat capacity.
When trying to analyse the effect of disorder they compared ordered and disordered samples, but also compared samples with different densities. They realized that past studies had compared typical glass to typical crystal but in doing so had compared low-density glass with high-density crystal, combing the disorder effect with a density effect.
In order to eliminate the effect of density and only see the effect of disorder it was necessary to compare the glassy and crystalline polymorphs having identical or very similar densities. A very clear and reproducible picture emerged: when a glass and a crystal with the same density are compared, the additional states are located at the same energies and provide the same heat capacity.
“It’s really very simple”, explains Chumakov, “atoms in an ordered state occupy a small volume whereas in a disordered state they usually occupy a bigger volume. The typical disorder state always has a smaller density than a typical ordered state. This in the only circumstance that makes the heat capacity for glasses bigger than for crystals.”
“The techniques developed here at the ESRF were crucial in reaching this conclusion, in combination with the very high energy resolution that is required”, adds Chumakov. “Nuclear Resonance beamline ID18 is the only place in the world where inelastic X-ray scattering measurements with an energy resolution of 0.5 meV are provided for users.”
References
Role of disorder in the thermodynamics and atomic dynamics of glasses, A.I. Chumakov, G. Monaco, A. Fontana, A. Bosak, R.P. Hermann, D. Bessas, B. Wehinger, W. A. Crichton, M. Krisch, R. Rüffer, G. Baldi, G. Carini Jr., G. Carini, G. D’Angelo, E. Gilioli, G. Tripodo, M. Zanatta, B. Winkler, V. Milman, K. Refson, M. T. Dove, N. Dubrovinskaia, L. Dubrovinsky, R. Keding, and Y. Z. Yue, Phys.Rev. Lett., scheduled for the January 17, 2014 issue.
www.dx.doi.org/10.1103/PhysRevLett.112.025502
Related articles:
Glass phase cracked - March 2013
Why chaos is warmer than order - May 2011
Top image: Glasses have a higher heat capacity than crystals not because of disorder, but because a disordered state occupies a larger volume and has a lower density. Credit: O. Tchoumakova.




