- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2016
- Structure of materials
- Nanometre-scale live view of catalyst degradation
Nanometre-scale live view of catalyst degradation
A novel sintering mechanism was revealed through high-energy grazing-incidence X-ray diffraction studies on the composition- and shape-dependent sintering behaviour of Pt-Rh nanoparticles during catalytic CO oxidation at near-ambient pressures. This mechanism was found to be suppressed for particles grown in their equilibrium shape. The latter was achieved by increasing the Rh-content inside the Pt-Rh alloy particles.
Catalysts are materials that increase the rate of a desired chemical reaction. They are widely employed in fuel cells, automotive exhaust converters and in the chemical industry where they are essential for the production of more than 90% of the world’s fine chemicals. To increase the surface area and accordingly the number of reaction sites, the catalyst materials (typically Pt, Rh, Pd and alloys thereof) are in the form of nanoparticles and are widely dispersed on highly branched oxide carriers, mainly Al2O3.
To improve catalyst efficiency and lifetime, an atomic-scale understanding of the processes that occur on these complex materials under reaction conditions is a prerequisite. Reaction conditions typically involve gas mixtures at elevated temperatures and ambient pressures. In recent years, much attention has been paid to the study of alloy nanoparticles, which, based on synergetic effects between the components, are promising systems for tailoring catalyst performance [1]. Sintering, i.e. the agglomeration of particles resulting in a loss of effective surface area, is viewed as a major cause of catalyst deactivation [2]. A better understanding of the process itself paves the way for improved sintering reduction.
At beamline ID15A, we performed a high-energy grazing-incidence X-ray diffraction study (E = 78.7 keV) which shed new light on the composition- and shape-dependent sintering behaviour of Al2O3(0001)-supported Pt-Rh alloy nanoparticles during CO oxidation at near-ambient pressures (p = 200 mbar; T = 550 K).
 |
|
Fig. 55: Experimental strategy allowing a correlation between the sample's catalytic activity and the atomic nanoparticle structure for different Pt-Rh alloy compositions. |
Figure 55 summarises the experimental scheme. The model catalyst consisted of stripes containing epitaxial nanoparticles of varying Pt-Rh compositions that could be probed individually by the collimated X-ray beam. It was mounted inside a beryllium dome acting as a flow reactor for catalysis. Sampling via a residual gas analyser (RGA) while detecting the scattered X-rays with a large area detector allowed the sample’s catalytic activity to be correlated with the particle structure of the different Pt-Rh compositions.
The use of high photon energies allowed a large area of reciprocal space within a single image to be mapped (see Figure 55), which included the particle (3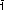 1) Bragg peak and its vicinity which contained finite height Laue oscillations (see inset of reciprocal space map). These intensity variations along the L-direction provided information about the particle height and the quantitative particle shape. Hence, the 2D snapshots served as a real-time probe to monitor the particle sintering.
1) Bragg peak and its vicinity which contained finite height Laue oscillations (see inset of reciprocal space map). These intensity variations along the L-direction provided information about the particle height and the quantitative particle shape. Hence, the 2D snapshots served as a real-time probe to monitor the particle sintering.
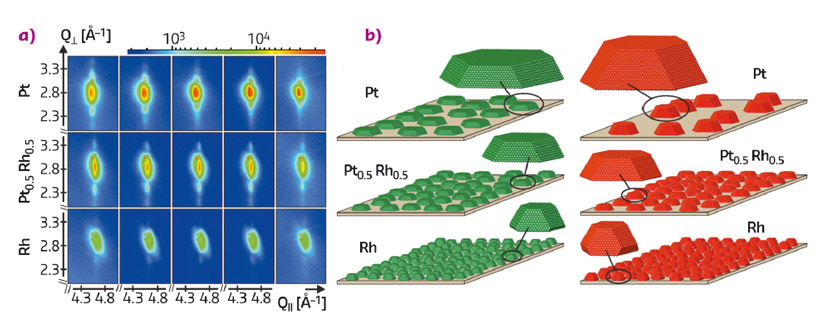
A sequence of these 2D reciprocal space maps taken during the transition to conditions of higher catalytic activity is shown in Figure 56a for selected Pt-Rh compositions. In the case of pure Pt, the Laue oscillations moved progressively towards the Bragg peak, indicating a strong particle height increase. The quantitative analysis revealed that, despite this strong vertical sintering, the initially very flat Pt particles maintained their diameter and accordingly adopted a more three-dimensional shape which proved to be very stable (see Figure 56b). X-ray reflectivity measurements showed that the atoms involved in this growth in height were rendered from the particles’ surroundings, as the particle height increase scaled with a decrease in the particle coverage on the sample surface.
With increasing Rh content, the initial particle shapes were found to be progressively more three-dimensional. Accordingly, the suppression of vertical sintering scaled with Rh content, as can be deduced from the Laue oscillations in Figure 56a, which almost maintained their position in the case of the Rh-rich particles.
We ascribe the particle shape change of the Pt-rich particles to a non-classical Ostwald ripening process that has not yet been described in literature. Therein, particles, kinetically trapped in an initially flat shape, use the energy released in the reaction to adopt a more compact equilibrium shape. We conclude that alloying with Rh results in the growth of stable equilibrium shapes and hence provides a possibility to reduce sintering.
Principal publication and authors
Tracking the shape-dependent sintering of platinum–rhodium model catalysts under operando conditions, U. Hejral (a,b,c), P. Mueller (a,b,c), O. Balmes (d,e), D. Pontoni (e) and A. Stierle (a,b,c), Nature Comm. 7, 10964 (2016); doi: 10.1038/ncomms10964.
(a) DESY, NanoLab, Hamburg (Germany)
(b) University of Hamburg, Fachbereich Physik, Hamburg (Germany)
(c) University of Siegen, Fachbereich Physik, Siegen (Germany)
(d) MAX IV Lab, Lund (Sweden)
(e) ESRF
References
[1] J.Y. Park et al., Nano Letters, 8, 673-677 (2008).
[2] C.H. Bartholomew, Appl. Catal. A Gen., 212, 17-60 (2001).