- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2016
- Matter at extremes
- Discovery of new high pressure iron oxides points to a large oxygen source in Earth's mantle
Discovery of new high pressure iron oxides points to a large oxygen source in Earth's mantle
Studies of iron oxides under extreme conditions reveal a possible novel mechanism of oxygen recycling in the Earth's interior with potentially great impact on geochemical processes.
Materials exposed to extreme conditions such as high pressures and high temperatures (HPHT) can undergo chemical reactions that are totally different from those known at ambient conditions. Recent revolutionary developments in the field of high pressure crystallography have extended the achievable range of pressures useable for single crystal studies by an order of magnitude, beyond 150 GPa. Quantitative in situ characterisation of chemical changes in materials heated to thousands of degrees at pressures of dozens of GPa used to be a dream for high pressure scientists, but now we have demonstrated that this is indeed possible. We have acquired high quality single-crystal X-ray diffraction data from samples in the Fe-O system using laser heated diamond anvil cells at third generation synchrotrons, in particular at the ESRF at beamline ID09A (now ID15B), and combined these results with those from the unique energy-resolved synchrotron Mössbauer spectroscopy at beamline ID18 that allowed a detailed characterisation of structural, chemical, and electronic processes under extreme conditions.
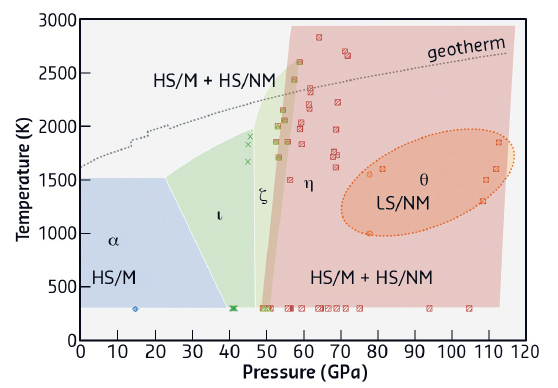
Our work is focused on the investigation of iron (III) oxide, Fe2O3, at pressures above 100 GPa and temperatures above 2500 K. Despite a strikingly simple chemical composition, this oxide undergoes a series of enigmatic structural and electronic transitions at elevated pressures and temperatures. We observed two transformational paths in Fe2O3 depending on the type of external stimuli applied. During room-temperature compression, Fe2O3 undergoes two phase transitions (to ξ-Fe2O3 at 54 GPa and θ-Fe2O3 at 67 GPa) accompanied by an iron spin crossover and a related abrupt change of the unit cell volume. The highest-pressure phase θ-Fe2O3, having Fe3+ in only a low spin state, can be observed to at least 100 GPa and is metastable. Moderate heating with a laser provokes its transformation to a η-Fe2O3 phase with a post-perovskite structure, which is stable down to at least 50 GPa; heating results in transformations of this phase first into ξ-Fe2O3 and then ι-Fe2O3. Figure 131 show these numerous phases and phase transformations, accompanied by changes in the electronic structure of Fe2O3.
 |
|
Fig. 131: Transformational phase diagram of Fe2O3. Legend: diamond - hematite (α-Fe2O3), triangles - distorted perovskite (ζ-Fe2O3), circles - Aba2 (θ-Fe2O3, probably metastable), squares - Cmcm post-perovskite (η-Fe2O3), crosses - Rh2O3-II type phase (ι-Fe2O3). Letters designate the electronic and magnetic state of iron atoms: HS – high-spin, LS – low spin, M – magnetic ordering, NM – absence of magnetic ordering. |
The most intriguing observation was that Fe2O3is chemically unstable at high pressures and temperatures: if heated at pressures above ~60 GPa, Fe2O3with the post-perovskite structure (η-Fe2O3) releases oxygen and forms a novel Fe5O7 compound. Inspired by this observation, we performed experiments on Fe3O4 and found that this iron oxide also decomposes to form the previously unknown Fe25O32 upon heating at pressures above ~70 GPa. Thus, it is firmly established that the chemistry of the Fe-O system at even moderate pressures is remarkably different from that which is known at ambient pressure.
 |
|
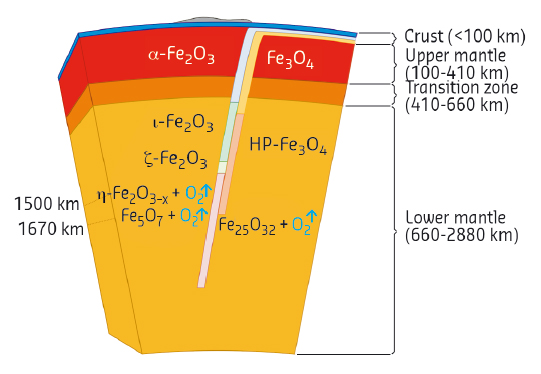
Fig. 132: Possible consequence of phase transitions of Fe2O3 and Fe3O4 in a BIF subducted to the lower mantle. |
The results of this work have important implications not only for fundamental high-pressure chemistry, but also for the understanding of the global rock development processes. Hematite (α-Fe2O3) and magnetite (Fe3O4) are major components of the so-called banded iron formations (BIFs) and ironstones, huge sedimentary rock formations occurring on all continents (and the main source of iron for our civilisation). BIFs may reach up to several hundred metres in thickness and hundreds of kilometres in length. Deposited in the world's oceans about two billion years ago, BIFs as part of the ocean floor are recycled into the Earth's interior by subduction to depths extending possibly to the core-mantle boundary region. According to the present study, hematite and magnetite would undergo numerous phase transformations upon subduction of BIFs into the lower mantle (Figure 132). At pressures above ~60 GPa, iron oxides (particularly η-Fe2O3) start to decompose, producing oxygen. Based on estimates of the amount of BIFs subducted into the Earth's mantle, the amount of oxygen produced by the formation of Fe5O7 alone can be as high as 8 to 10 times the mass of oxygen in the modern atmosphere. An oxygen-rich fluid could either locally oxidise surrounding materials or pass to the transition zone, or even to the upper mantle, thus shifting Fe2+/Fe3+ equilibria in silicate minerals and greatly raising the oxygen fugacity in this region. In any case, our study suggests the presence of an oxygen-rich fluid in the deep Earth's interior that can significantly affect geochemical processes by changing oxidation states and mobilising trace elements.
Principal publication and authors
Structural complexity of simple Fe2O3 oxide at high pressures and temperatures, E. Bykova (a,b), L. Dubrovinsky (a), N. Dubrovinskaia (b), M. Bykov (b), C. McCammon (a), S.V. Ovsyannikov (a), H.-P. Liermann (c), I. Kupenko (a,d), A.I. Chumakov (d), R. Rüffer (d), M. Hanfland (d) and V. Prakapenka (e), Nature Communications 7, 10661 (2016); doi: 10.1038/NCOMMS10661.
(a) Bavarian Research Institute of Experimental Geochemistry and Geophysics, Universität Bayreuth (Germany)
(b) Laboratory of Crystallography, Universität Bayreuth (Germany)
(c) Photon Sciences, Deutsches Elektronen-Synchrotron, Hamburg (Germany)
(d) ESRF
(e) Center for Advanced Radiation Sources, University of Chicago, Argonne (USA)



