- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2016
- Matter at extremes
- Stable subnanometric platinum catalyst characterised by XAS
Stable subnanometric platinum catalyst characterised by XAS
Single metal atoms and clusters have unique catalytic properties compared with conventional nanoparticulate catalysts. In this work, subnanometric Pt species (single atoms and clusters) were encapsulated within a three-dimensional zeolite MCM-22 during its synthesis from a two-dimensional precursor. X-ray absorption spectroscopy provided an insight into the local environment of the Pt species in the Pt@MCM-22 complex.
Single metal atoms and metal clusters have attracted much attention thanks to their efficacy as heterogeneous catalysts [1]. However, the generation of stable single atoms and clusters on a solid support is still challenging [2]. Here we describe a new strategy for the generation of single Pt atoms and Pt clusters with exceptionally high thermal stability, formed within purely siliceous MCM-22 during the transformation of a two-dimensional precursor of the zeolite into the three-dimensional structure. This platinum zeolite complex remains stable even on heating in air to 540°C.
 |
|
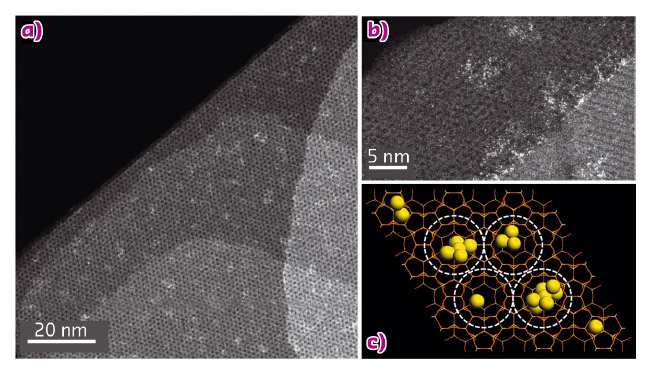
Fig. 122: Atomic structure of Pt@MCM-22 sample. a) and b), HAADF-STEM image of Pt@MCM-22. The bright dots correspond to subnanometric Pt species, including single atoms and clusters with a few atoms. c) Schematic illustration of Pt@MCM-22 in a 'top-down' view along the c axis. Pt clusters and individual Pt atoms are located in the surface cups, cavities and 12-MR supercages. |
As shown in Figure 122, the subnanometric Pt species are finely dispersed in the MCM-22 crystallites. The size of the Pt species and their location in the crystallites was determined with the help of aberration-corrected electron microscopy. The Pt atoms and clusters are located both in the surface “cups” of MCM-22 and within the zeolite framework. In particular, a large proportion of the subnanometric Pt species are located in the internal space of the structure.
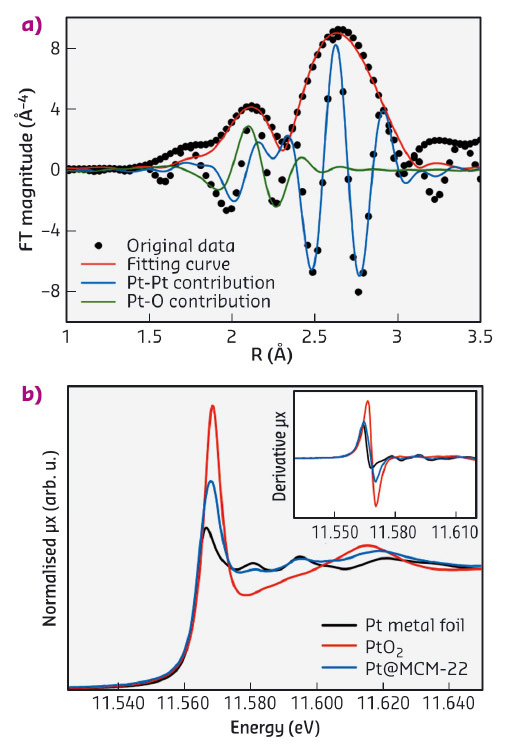
A Pt@MCM-22 sample has been studied at beamline BM23 using X-ray absorption spectroscopy (XAS) to examine the local environment of Pt and to estimate the coordination number of Pt species. The Fourier transform of extended X-ray absorption fine structure (EXAFS) spectra of Pt@MCM-22 and the Pt and PtO2 reference are shown in Figure 123a. Considering the first shell, the Pt@MCM-22 spectrum is dominated by a peak centred around 2.65 Å with a smaller contribution at 2.08 Å (both distances are not phase corrected). Comparing with reference samples, the stronger contribution can be assigned to Pt-Pt bonds in Pt clusters. The weaker peak can be assigned to Pt-O bonds between Pt and the zeolite frameworks. The presence of Pt-O bonds in the Pt@MCM-22 sample has also been confirmed by the X-ray absorption near edge structure (XANES) spectrum of Pt@MCM-22. In Figure 123b, the curve shape for the Pt@MCM-22 sample looks like Pt(0) (first derivative and oscillations around 11580 and 11595 eV) but its white line intensity is higher, indicating the presence of Pt-O bonds. Based on the fitting results of the EXAFS spectrum, the coordination number of Pt in Pt@MCM-22 is ca. 4.7, which would correspond to Pt clusters of less than 0.7 nm (i.e. less than 13 atoms), assuming that Pt clusters show cubo-octahedral shape. The information obtained from electron microscopy and XAS support the hypothesis that the subnanometric Pt species are encapsulated by the zeolite structure.
 |
|
Fig. 123: Characterisation of Pt@MCM-22 with XAS. a) Fourier transform of k3 -weighted EXAFS spectrum of the Pt@MCM-22 sample (not phase corrected). The fitting curves for Pt–O and Pt–Pt contribution are also presented. b) XANES spectrum of Pt@MCM-22, with the first-order derivative spectrum given in the inset. |
These stable Pt species confined within internal framework cavities show size-selective catalysis for the hydrogenation of light olefins. Pt@MCM-22 shows higher activity in the hydrogenation of propylene rather than isobutene. Furthermore, high-temperature oxidation-reduction treatments result in the growth of encapsulated Pt species into small nanoparticles in the approximate size range of 1 to 2 nm. The stability and catalytic activity of the encapsulated Pt species is also reflected in the dehydrogenation of propane to propylene. Therefore, this new method provides a means to generate highly stable subnanometric metal catalysts in pure siliceous zeolites for high-temperature catalytic reactions.
Principal publication and authors
Generation of subnanometric platinum with high stability during transformation of a 2D zeolite into 3D, L. Liu (a), U. Díaz (a), R. Arenal (b,c), G. Agostini (d), P. Concepción (a) and A. Corma (a,e), Nature Materials 16, 132–138 (2017); doi: 10.1038/nmat4757.
(a) Instituto de Tecnología Química, Universitat Politècnica de València-Consejo Superior de Investigaciones Científicas (UPV-CSIC), Valencia (Spain)
(b) Laboratorio de Microscopias Avanzadas, Instituto de Nanociencia de Aragon, Universidad de Zaragoza (Spain)
(c) ARAID Foundation, Zaragoza (Spain)
(d) ESRF
(e) King Fahd University of Petroleum and Minerals, Dhahran (Saudi Arabia)
References
[1] M. Boronat et al., Acc. Chem. Res. 47, 834-844 (2014).
[2] A. Corma et al., Nat. Chem. 5, 775-781 (2013).



