- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2016
- Industrial research
- AMPK: a multiprotein drug target unravelled
AMPK: a multiprotein drug target unravelled
The quest for drug development against several diseases has led CRELUX/WuXi AppTec, a company providing drug discovery solutions for global pharma, to analyse an AMPK (AMP-activated protein kinase) at the structural biology beamlines.
AMPK (AMP-activated protein kinase) is a Ser/Thr kinase composed of two regulatory subunits and a catalytic subunit that together as a complex regulates the levels of energy in the cells. This complex is evolutionarily conserved and ubiquitously expressed. There are a total of 12 possible isoforms, which are distributed in the human body in a tissue-specific manner. For example, one isoform is highly expressed in skeletal muscles and a different isoform is more specific to heart, brain or liver. All in all, AMPK senses the energy levels of the cells (in the form of the so-called ATP) and allows upstream signals to activate it, in response to external nutritional stress. AMPK substrates are involved in lipid metabolism, autophagy, mitochondrial biogenesis, and in the maintenance of glucose homeostasis. Therefore, this complex is a highly promising therapeutic drug target against diabetes, obesity, Wolff-Parkinson-White Syndrome, cancer, and ageing.
There are, however, several challenges in the generation of soluble and stable complexes, and this is one of the many reasons why few X-ray structures are available, especially at resolutions suitable to drive drug discovery efforts. Firstly, it is very complicated to be able to make crystals of the complex with the activated compound bound to it. Another obstacle is the extreme sensitivity of the tiny crystals to radiation damage.
 |
|
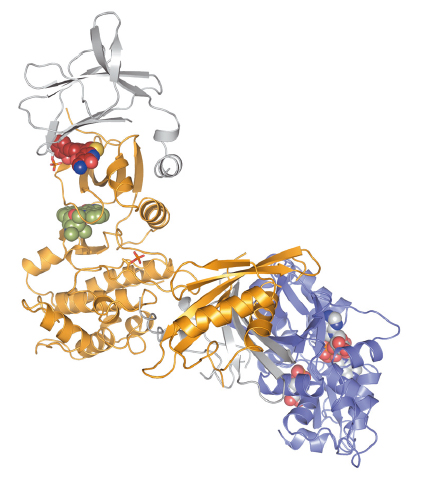
Fig. 146: The unpublished structure shows an allosteric activator bound to a human AMPK isoform at 2.9 angstrom resolution. Credits: CRELUX. |
Scientists from CRELUX/ WuXi AppTec, a company that is a worldwide expert in premium drug discovery solutions for global pharma, biotech and research organisations, sent crystallised AMPK samples to beamline ID30A-1. Because of the complexity of the project, they needed a powerful beamline, a state-of-the-art detector and a skilled scientist in-house to carry out the experiment. They managed to solve the structure of the complex at a resolution of 2.9 angstroms (Figure 146), which was enough for CRELUX/ WuXi AppTec to see the detailed chemical environment of the compound in the complex binding site. This corresponds to one of the highest resolution structures published so far for any AMPK isoform.
The work at the ESRF will help CRELUX to support their clients in the discovery and development of novel and more specific drugs that can influence AMPK activity in the cell and, as a result, adjust the energy balance in disease affected organs. Debora Konz Makino, Lab Head at CRELUX/ WuXi AppTec, explains: ”Our long-standing collaboration with the ESRF has greatly contributed to the success of most of our clients’ projects in the early stages of drug discovery. The ESRF provides not only cutting edge infrastructure, but also excellent scientific support for X-ray data collection of biological macromolecules. We at CRELUX highly appreciate the ESRF’s prompt and open communication.”



