- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2016
- Complex systems and biomedical sciences
- How sea cucumbers stiffen and soften their tissues
How sea cucumbers stiffen and soften their tissues
The mutable collagenous tissue found in sea cucumbers and starfish is unique among connective tissues because it can change its mechanical state quickly. However, how it achieves this feat is not known. Using synchrotron X-rays to study collagen deformation in situ, the mechanical change is explained by a change in the gel-like matrix between collagen fibrils.
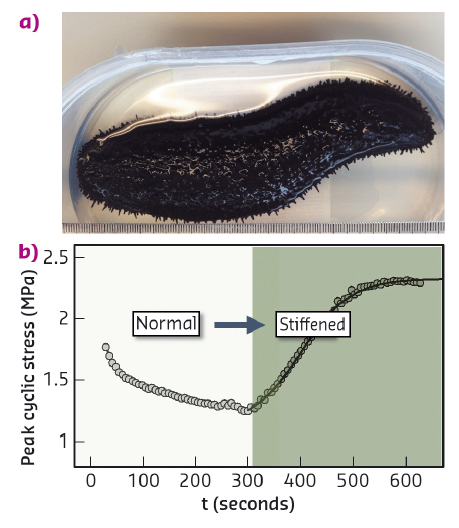
Collagen is a core building block of both vertebrate and invertebrate connective tissues. In mechanically critical tissues like tendons, skin and heart valves, the fibrillar composite architecture – including collagen, proteoglycans, fibrillin and other proteins and water – is adapted to the needed in vivo mechanical response. These mechanical properties are usually constant over short periods of time (seconds) and vary only on much longer timescales (days or months). However, one remarkable exception is the "mutable collagenous tissue" (MCT) which is found in echinoderms – such as starfish, sea cucumbers and brittle stars (Figure 98a). Such animals use MCT to rapidly change their mechanical properties in a few seconds, for example softening their body wall to escape predators, emit sticky tubules from their gut as a defence mechanism or engage in locomotion in an energy efficient manner (Figure 98b). Understanding how MCT works sheds light on the remarkable adaptation in a highly successful group of animals, the echinoderms, and it also provides insight into the development of biomaterials with changeable mechanical properties.
 |
|
Fig. 98: a) Black sea cucumber (Holothuria edulis). b) Increase in peak cyclic force on MCT when changing the state of the tissue from standard to stiff (with addition of KASW) showing nearly 80% increase in stress. |
At the ultrastructural level, MCT consists of spindle-shaped collagen fibrils in an interfibrillar matrix of proteoglycans and noncollageneous proteins like tensilin and stiparin, which along with fibrillin-rich microfibrils comprise the extracellular matrix [1]. Embedded inside MCT are effector cells known as juxtaligamental cells (JLCs) that are innervated and controlled by the nervous system. Currently the proposed mechanisms enabling MCT in echinoderm tissue suggest that the JLCs play a role by releasing proteins that crosslink the fibrils, but to date there have been no direct experimental studies of the ultrastructural mechanisms.
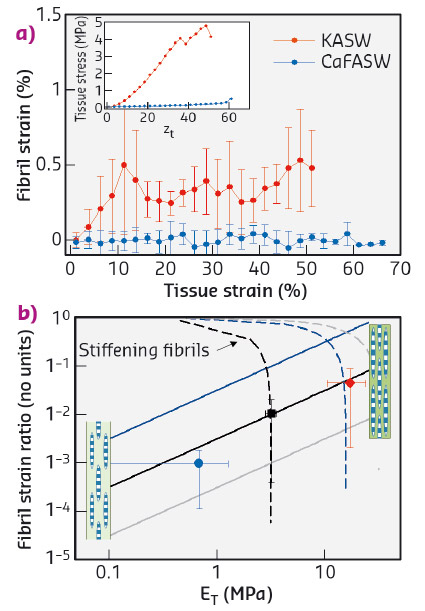
To resolve the fibrillar-level mechanics, we used time-resolved synchrotron small angle X-ray diffraction (SAXD) combined with in situ mechanical testing of MCT in different states of mechanical activation (via ionic stimulation). Collagen fibrils have a banded pattern along the fibril leading to a series of Bragg peaks in the meridional SAXD pattern. Therefore, real-time acquisition of stress-induced changes in fibrillar mechanical strain show up as shifts in the meridional Bragg peaks [2]. We carried out in situ SAXD measurements at beamline ID02 to determine fibril strain and orientation. Mechanically stimulated MCT (incubated in potassium rich artificial sea water or KASW) showed a clear increase of fibril strain with applied tissue strain (Figure 99a). In contrast, MCT in the soft state (incubated in calcium free sea water or CaFASW) exhibited almost no increase in fibril strain with tissue strain. Concurrently, angular SAXD intensity distributions showed that fibrils in stiffened MCT transitioned quicker (relative to softened MCT) to align along the external loading direction.
 |
|
Fig. 99: a) Fibril strain increases with tissue strain for KASW-treated stiff MCT whilst showing no increase in CaFASW treated soft MCT (inset shows macroscopic stress-strain plots for the two cases). b) Using a fibril-level model of force transfer at the nanoscale in MCT, the experimental data (solid symbols) for the fibril strain fraction follow the trend for increasing interfibrillar matrix stiffness (solid lines) rather than stiffening collagen fibrils (dashed lines). Inset: schematics of interfibrillar sliding (left) and fibrillar stretching (right). |
We explain these findings by considering how shear stresses in the interfibrillar matrix of proteins and glycosaminoglycans enable tensile stress and strain in the fibril. By increasing the interfibrillar matrix stiffness, both the tissue modulus and the amount of fibril strain increase (Figure 99b), whilst if the fibrils become more resistant to stretching, the two parameters are oppositely correlated. Our results clearly show that the mechanism for stiffening in echinoderm MCT is due to mechanical changes in the matrix between fibrils rather than changes in the fibril properties.
These results provide the first direct nanoscale evidence for the mechanism of rapid mechanical changes in echinoderm MCT. By demonstrating the importance of interfibrillar matrix proteins in this process, the work opens the way to testing the function of specific proteins (e.g. tensilin) in mediating the mutability mechanism. Understanding how collagenous matrices could soften or stiffen reversibly may have applications in developing treatments to combat hardening of tissues like skin in ageing.
Principal publication and authors
Interfibrillar stiffening of echinoderm mutable collagenous tissue demonstrated at the nanoscale, J. Mo (a), S. Prévost (b), L.M. Blowes (c), M. Egertová (c), N.J. Terrill (d), W. Wang (a,e), M.R. Elphick (c) and H.S. Gupta (a,e), PNAS 113, E6362-E6371 (2016); doi: 10.1073/pnas.1609341113.
(a) Queen Mary University of London, School of Engineering and Material Science, London (UK)
(b) ESRF
(c) Queen Mary University of London, School of Biological and Chemical Sciences (UK)
(d) Diamond Light Source, Didcot (UK)
(e) Institute of Bioengineering, Queen Mary University of London (UK)
References
[1] I.C. Wilkie, Mutable Collagenous Tissue: Overview and Biotechnological Perspective. In: Echinodermata (eds), Springer Berlin Heidelberg (2005).
[2] A. Karunaratne et al., Bone 84, 15-24 (2016).



