- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2014
- Structure of materials
- Time-resolved X-ray diffraction with enhanced sensitivity
Time-resolved X-ray diffraction with enhanced sensitivity
X-ray diffraction (XRD) is a powerful method to determine the structure and the structural changes of materials. An advantage of XRD is that it allows the observation of all XRD visible phases simultaneously within a complex multiphase material. When using synchrotron radiation, the information is obtained in a snapshot, i.e. with high time resolution. In the case of supported precious metal based catalysts, used for example to control the emissions of automotive engines, XRD provides information on the precious metal particles and the metal oxide support(s). Chemical reactions at the heart of the abatement of pollutants (NOx, CO and hydrocarbons) occur mainly on the precious metal nano-particles. The function of the metal oxide is to provide stability against severe reaction conditions. For example, CeO2 is responsible for maintaining metal nano-particles in an oxidised state, thus retarding their growth which otherwise leads to loss of the catalytic function. Strongly X-ray absorbing materials like CeO2 are difficult to analyse by X-ray absorption spectroscopy (XAS) with high time resolution. On the one hand, the strong absorption of X-rays by the metal oxides hinders detection of the low loaded active metals (Pt, Pd, Rh) and fluorescence based detection is often required. As a result, high time resolution is still an issue. High energy XRD is suitable to circumvent the inherent problems of XAS but is still limited by its intrinsic detection limit. Metal nano-particles are visible by XRD only if dispersion is low and the metal content high. Typically, detection is limited to ca. 3 nm particle size for high loaded metals.
At beamline ID15B (now ID31), we have used high energy XRD in combination with a modulation approach and lock-in amplification based on phase sensitive detection (PSD) [1] to follow redox processes on technologically relevant materials. A 2 wt% Pd/Ce0.6Zr0.4O2 catalyst is subjected to repeated and equally long pulses of CO and O2 at 573 K while XRD acquisition is performed at 500 ms/pattern. A combined XRD/DRIFTS/MS setup was used for this purpose [2]. After averaging, the set of time-resolved XRD patterns was analysed by PSD, which provided a new dataset containing information only on the species that changed dynamically during the modulation experiment.
 |
|
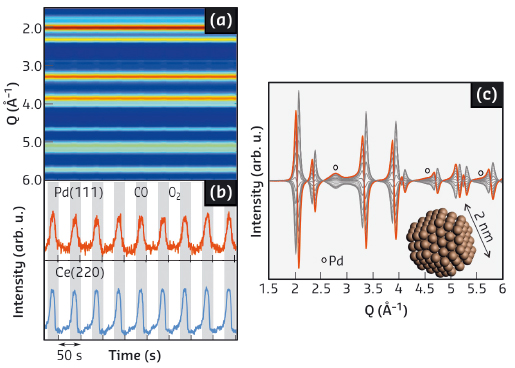
Fig. 135: Time-resolved XRD data. a) Colour map representation. b) Temporal responses of the XRD reflections. c) Phase-resolved data. |
Figure 135 shows that the time-resolved XRD data contain little or no information on any structural change induced by the modulation experiment. Features that could be associated with Pd or PdO nano-phases are absent as expected from the low metal loading and the absence of long range order that are at the base of the peculiar catalytic activity of such nano-entities. In marked contrast, the corresponding phase-resolved data obtained by PSD show two sub-sets of reflections signifying that structural changes occurred during the experiment. The first one is characterised by a differential profile (both by intensity and 2θ variation) and is associated with expansion and contraction of the Ce component upon reduction of Ce4+ (in CO) and oxidation of Ce3+ (in O2). The very small (0.11%) and reversible change of d spacing is associated with the capacity of CeO2 to exchange oxygen with the environment under reducing conditions. The second sub-set entails only an intensity change with equally reversible behaviour. The position of this sub-set of reflections coincides with the XRD pattern of fcc Pd. Estimation of the crystallite size by the Scherrer formula provides Pd particles of <2 nm that become visible only after application of PSD. This size estimate is supported by simulation of particles in the range 1-5 nm. The temporal behaviour of the two datasets demonstrates that evolution of the structural change within Ce0.6Zr0.4O2 and reduction-oxidation of the Pd component occur quasi simultaneously. Also, the function of Ce3+/Ce4+ becomes clear in the appearance of the Pd(111) reflection in the PSD data. This shows that reduction of Pd is retarded until the very end of the reducing pulse as a result of the intimate contact of the Ce0.6Zr0.4O2 and Pd phases.
Therefore, we have demonstrated that a greater detail of the redox kinetics of a complex material can be obtained by the combination of high energy XRD, the modulation approach and PSD. Subtle changes associated with metal nano-particles can be made visible using a bulk analysis method. Such enhanced insights should be achievable in all systems that involve functional materials where reversible cycling is possible for one or more of the intrinsic process variables.
Principal publication and authors
D. Ferri (a), M.A. Newton (b), M. Di Michiel (b), G.L. Chiarello (c,d), S. Yoon (c) Y. Lu (c) and J. Andrieux (b,e), Angew. Chemie Int. Ed. 53, 8890–8894 (2014).
(a) Paul Scherrer Institut (Switzerland)
(b) ESRF
(c) Empa, Swiss Federal Laboratories for Materials Science and Technology (Switzerland)
(d) Present address: Università degli Studi di Milano (Italy)
(e) Present address: Université de Lyon (France)
References
[1] D. Baurecht, U.P. Fringeli, Rev. Sci. Instr. 72, 3782 (2001); D. Ferri, M.A Newton, M. Di Michiel, S. Yoon, G.L. Chiarello, V. Marchionni, S.K. Matam, M.H. Aguirre, A. Weidenkaff, F. Wen and J. Gieshoff, PCCP 15, 8629 (2013).
[2] M.A. Newton, M. Di Michiel, A. Kubacka and M. Fernàndez-Garcià, J. Am. Chem. Soc. 132, 4540 (2010).



