- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2014
- Dynamics and extreme conditions
- (N2)3Ne7: a high pressure van der Waals insertion compound
(N2)3Ne7: a high pressure van der Waals insertion compound
Through increased pressure, two different atomic or molecular species that interact via weak van der Waals forces can auto-organise to form stoichiometric compounds, called van der Waals compounds. The effect of pressure is to modify the microscopic interactions and stabilise new structures. Remarkably, none of these van der Waals compounds have been observed at low temperatures in cryocrystals, which makes pressure a powerful driving force for self-assembly of molecular compounds.
Most of the compounds that have been observed so far under pressure, such as NeHe2, Ar(H2)2, CH4(H2)2, Xe(O2)2 crystallise in the form of Laves phases, i.e. analogous to intermetallic compounds and colloidal systems, the stability of which is explained in terms of binary crystals of hard sphere like particles. However, the van der Waals forces can also be anisotropic which, combined with packing constraints, may lead to more exotic structures. For example, in pure N2, the quadrupole-quadrupole interaction between the N2 molecules is responsible for a rich polymorphism under pressure.
In this study, we have discovered a new type of van der Waals compound in the N2-Ne system at high pressure. This compound has the stoichiometry (N2)6Ne7, with the structure of a clathrate-like insertion compound. It is the first van der Waals clathrate ever observed and it could open a route for the development of a new family of host-guest systems in high pressure molecular systems.
To unveil the existence of this stoichiometric compound, we performed a detailed measurement of the N2-Ne binary phase diagram, at ambient temperature, by studying 16 different concentrations, as shown in Figure 85. The fluid mixtures were loaded in a diamond anvil cell. A ruby gauge was used to measure the pressure of the sample. The different phases were identified by Raman spectroscopy of the vibration modes of the N2 molecule.
 |
|
Fig. 85: Binary phase diagram of the N2-Ne mixture at 296 K, plotted as pressure vs Ne concentration. S1 and S2 are the pure solids and F is the fluid phase. Inset: Photograph of a single crystal of (N2)6Ne7 surrounded by a solid-solid phase separation, in the diamond anvil cell. |
The sample was compressed to several gigaPascals, and the solidification was observed visually through the diamond anvils, using a microscope. The pressure at which the solid crystal disappears in the liquid is the melting pressure for a given concentration (red dots in Figure 85). The particular form of the binary phase diagram reveals the presence of a stoichiometric compound at 54 mol% concentration of neon, corresponding to the formula (N2)6Ne7. At this pressure, congruent melting was observed and a solid with hexagonal structure was identified, as shown in the inset of Figure 85.
 |
|
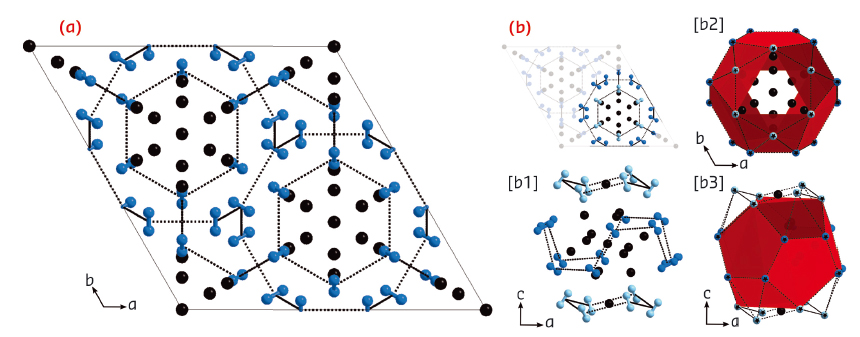
Fig. 86: Crystal structure of the (N2)6Ne7 compound. Neon and nitrogen are represented by black and blue spheres respectively. The red planes show the surfaces supported by the centres of the N2 molecules. |
The structure of this compound was determined by single-crystal angular dispersive X-ray diffraction at beamline ID09A. The synthesis of a high quality single crystal, and the use of a high brilliance, monochromatic synchrotron beam optimised for diffraction at extreme conditions using diamond anvil cells was essential to the determination of the structure of (N2)6Ne7. The diffraction signal was composed of 225 reflections, which were indexed to find that the structure is hexagonal with symmetry R-3m. The positions of the atoms were determined by direct methods and refined. The structure is shown in Figure 86 The N2 molecules surround the Ne atoms, forming cages with pentagonal faces, similar to the structures of clathrates. The fact that van der Waals interactions could drive the self-organisation of such a complex structure was unexpected. The prediction of this structure could be a challenging test for first principle calculations.
Such an exotic structure could also be used to stabilise new compounds synthesised at high pressure. For example, pure nitrogen was shown to polymerise at high pressure [1]. This polymeric single-bonded form is known to be a dense high-energy material that could be used as fuel, explosive or propellant. But it has not yet been recovered at ambient pressure. Constraining the geometry of the nitrogen molecules in such van der Waals compounds could change the conditions of polymerisation and the recovery of the polymeric compound at lower pressure. It could also serve as a pathway for the observation of other allotropic structures predicted theoretically, among which is an intriguing diamondoid structure formed by N10 cage units [2]. The compression of this compound to the megabar range is underway.
Principal publication and authors
T. Plisson (a), G. Weck (a) and P. Loubeyre (a), Phys. Rev. Lett. 113, 025702 (2014).
(a) CEA, DAM, DIF, Arpajon (France)
References
[1] M.I. Eremets, A.G. Gavriliuk, I.A.Trojan, D.A. Dzivenko and R. Boehler, Nat. Mater. 3, 558 (2004).
[2] X. Wang et al, Phys. Rev. Lett. 109, 175502 (2012).



