- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2013
- Structure of materials
- Mixed-linker hybrid superpolyhedra for large-pore iron(III) carboxylate metal–organic frameworks
Mixed-linker hybrid superpolyhedra for large-pore iron(III) carboxylate metal–organic frameworks
Metal organic frameworks (MOFs) are attracting great interest for their potential in various applications ranging from gas capture to storage to catalysis. While most of the solids reported in the literature are based on M2+ cations (e.g. Zn2+, Cu2+, Ni2+), Mn+ with n ≥ 3 (Fe3+, Al3+, Cr3+, Zr4+, Ti4+) species have been shown to induce higher chemical stabilities [1], which is often a prerequisite for further industrial applications. Among these cations, Fe3+ deserves special attention due to its low toxicity, which makes the resulting solids highly suitable for bio-applications such as drug release [2]. While the structural diversity is mainly induced by building structures with more and more complex organic ligands, an alternative consists of the use of a mixture of simple ligands with different symmetries. Applying such a strategy with linear dicarboxylic and planar tricarboxylic ligands, we were able to generate two series of original Fe3+ MOFs built up from hybrid polyhedra. Contrary to the case of the n = 2 counterparts, MOFs based on n ≥ 3 are very often produced in a polycrystalline form, and as a consequence, their structures cannot be solved using laboratory single crystal X-ray diffractometers. Taking advantage of the versatility of the image-plate detector available at BM01A, the Swiss-Norwegian beamline, we solved the structures by a combination of single crystal and powder diffraction analyses, assisted with molecular simulations. Using a mixture of 1,4-benzenedicarboxylic acid (H2-bdc) (Figure 136b) and 1,3,5-tris(4-carboxyphenyl)benzene (H3-btb) (Figure 136c), we have generated two solids, both based on the same inorganic unit, which consists of three corner sharing FeO6 octahedra surrounded by six carboxylate groups (Figure 136a). Organic and inorganic units assemble to define hybrid super-polyhedra, built up from Fe trimers at their corner and ligands on their edges (when ditopic) or faces (when tritopic). The structure of the first solid (later called MIL-143, MIL stands for Materials Institut Lavoisier), which was isolated at shorter reaction time, was solved from powder diffraction. Its cubic structure consists of an alternation of two types of hybrid super-tetrahedra, one based on bdc and identical to those found in the single ligand solid MIL-101 [3], and the second one based on btb (Figure 136). This defines an extended ß-cristobalite topology and mesoporous cages (Figure 136) leading to a high surface area (SBET above 2000 m2.g-1). The structure of the second phase (denoted MIL-142), which was isolated at longer reaction time, was solved from single crystals. It crystallises in a rhombohedral system with an unusually large c parameter (~ 95 Å), and is constructed from a single type of hybrid super-octahedron (SO). The latter is built up from a mixture of ligands, with four faces occupied by btb linkers, and one face defined by three bdc ligands, the remaining faces not being occupied (Figure 136). Such SOs assemble to define a ReO3 network type, the whole structure being ultimately built from two such interwoven networks, leading to micropores (~ 7-10 Å) and a surface area around 1500 m2.g-1.
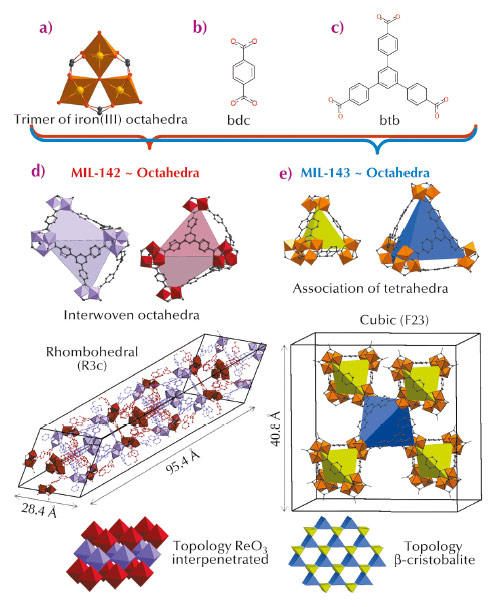 |
|
Fig. 136: (a) Trimers of iron(III) octahedra. (b) Terephthalate linker. (c) 1,3,5-tris(4-carboxyphenyl)benzene linker. (d) & (e) Representation and topology of MIL-142 and MIL-143, respectively. Hydrogen has been omitted for clarity. |
In a last step, using longer, functionalised dicarboxylate ligands, the MIL-142 and MIL-143 series were extended through an isoreticular approach. The structure of these solids was determined through a computational assisted approach (experimental unit-cells combined with simulated structures derived from the known ones); analyses included the impact of the functionalisation on the pore size and shape.
Principal publication and authors
H. Chevreau (a), T. Devic (a), F. Salles (b), G. Maurin (b), N. Stock (c) and C. Serre (a), Angew. Chem. Int. Ed., 52, 5056-5060 (2013).
(a) Institut Lavoisier de Versailles, CNRS, Université de Versailles St-Quentin en Yvelines (France)
(b) Institut Gerhardt, CNRS, ENSCM, Université de Montpellier (France).
(c) Institut fur Anorganische Chemie, Christian-Albrechts-Universität, Kiel, (Germany)
References
[1] J.J. Low, A.I. Benin, P. Jakubczak, J.F. Abrahamian, S.A. Faheem and R.R. Willis, Journal of the American Chemical Society 131, 15834-15842 (2009).
[2] P. Horcajada, T. Chalati, C. Serre, B. Gillet, C. Sebrie, T. Baati, J.F. Eubank, D. Heurtaux, P. Clayette, C. Kreuz, J.-S. Chang, Y.K. Hwang, V. Marsaud, P.-N. Bories, L. Cynober, S. Gil, G. Férey, P. Couvreur and R. Gref, Nat. Mater. 9, 172-178 (2010).
[3] G. Férey, C. Mellot-Draznieks, C. Serre, F. Millange, J. Dutour, S. Surblé and I. Margiolaki, Science 309, 2040-2042 (2005).



