- Home
- News
- General News
- New method to reveal...
New method to reveal multiple cell types in complex tissues unveiled
01-09-2023
Scientists from Universität Zürich and ETH Zürich, in collaboration with the ESRF, have developed a new molecular contrast bio-imaging method to overcome the limitations of current tagged microscopy techniques. The method offers non-destructive, multi-scale, high-speed imaging for 20 molecular markers at once, to characterise multiple features in complex tissues. The work was done at ID15A, which uses high-energy X-rays that are unusual for biological studies but have distinct advantages.
When cancer appears in a tissue, the presence and location of immune cell subtypes, cancer cells and resident structural cells defines the disease subtype and likelihood a patient will respond to a particular treatment. For example, in breast cancer, the prevalence of particular molecular markers such as the HER2 receptor indicates if a patient is likely to respond to Herceptin treatment. The state of immune cells in a tumour indicates whether a patient is likely to respond to immunotherapy.
Pathologists image molecular features in biological tissues to dissect tissue pathology. Molecular markers help identify specific cell types, cell states and reveal secreted factors that influence disease progression. As our understanding of diseases has advanced, imaging methods have sought to reveal more tissue features in parallel for a more complete understanding of how tissue function emerges from its component parts. However, current imaging methods have limitations in terms of speed, resolution and the ability to measure multiple markers without damaging samples. Much of this is due to the physical limits of light and spectral overlap of probes used in traditional light microscopy methods.
Now, a team at the University of Zürich and ETH Zürich (Switzerland), in collaboration with scientists at the ESRF’s ID15A beamline, has developed a new method to bring tagged molecular contrast to X-ray microscopy and analyse molecular features across tissues down to cellular level resolution.
In the new technique, called “Multi-element Z-tag X-ray fluorescence”, the researchers bind high-Z metals to antibodies, which are then used to stain tissues. The metal-bound antibodies have a strong binding affinity for specific molecules in tissue, and so target the metal to molecular features that can then be imaged by X-ray fluorescence.
|
|
|
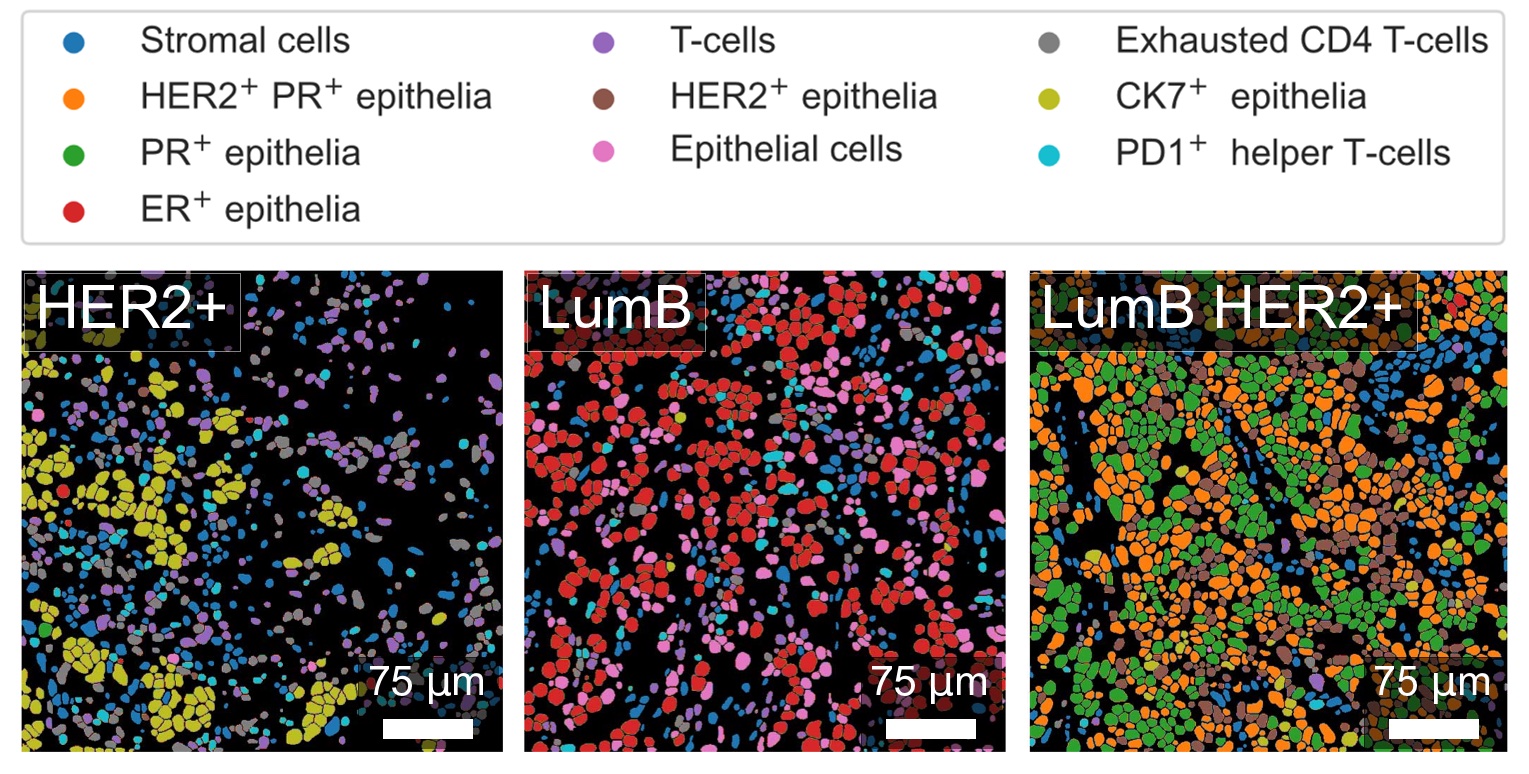
Different cell types identified in three different human breast cancer subtypes, including human epidermal growth factor receptor 2 positive (HER2), luminal B, and luminal B HER2 positive samples. Cell types were identified based on expression of multiple molecular markers that were labelled with heavy metals and mapped by X-ray fluorescence on beamline ID15A. Credits: M. Strotton. |
“We can look at up to 20 markers at the same time across large tissue samples, at high speed and in a non-destructive way, even if the markers have low expression,” explains Merrick Strotton, first author of the publication. “Once we have looked at the area in general and we have spotted the cells that we are interested in, we can then zoom into specific regions for more sensitive scans that reveal finer cell features, or low expression but important molecular markers,” he adds.
To demonstrate the method, the team focused mainly on human breast cancer tissue, tonsil and appendix samples. “Thanks to the high-flux and high-energy focusing optics available at ID15A, with this first demonstration of the approach we were already able to achieve very fast imaging speeds, competitive with comparable well established and optimised imaging methods.”
For Marco Di Michiel, scientist in charge of ID15A, this was an unusual experiment: “This beamline is a materials chemistry beamline; it is very rare to have biological samples like cancer tissues as we have a very high-energy beam. But in this case, because of the presence of high-Z elements, this is precisely the beamline the team needed.” There was no risk of the high-energy beam destroying the samples: the low-Z elements that comprise biological samples are essentially transparent to the high-energy beam, which interacts only with high-Z elements used as tags. Other practical advantages are that samples can be analysed in air, keeping the set-up relatively simple.
The method is similar to mass-spectrometry but without destroying the sample, explains Tsuyoshi Hosogane, another first author of the study. He says: “A clear advantage is that because we don’t destroy the samples, we can do follow-up complementary scans to maximise the information we derive from previous tissue samples.” And Strotton adds: “The really exciting thing about this method is that by bringing tagged molecular contrast agents to X-ray microscopy, there are clear paths to using tissue penetrant, short wavelength X-rays to achieve high-speed, high-resolution or even 3D imaging of large numbers of molecular features in parallel.”
Currently, this work is academic but in the future it might translate into medical applications. “This paper is a very robust demonstration that the method works. We have unlocked the potential of a new imaging technique that can have an impact on our understanding of complex pathologies,” concludes Professor Bernd Bodenmiller.
Reference:
Strotton, M. et al, Nature Methods, 31 August 2023. DOI: 10.1038/s41592-023-01977-x.
Text by Montserrat Capellas Espuny
Top image: Different cell types identified in human breast cancer luminal B HER2 positive samples. Cell types were identified based on expression of multiple molecular markers that were labelled with heavy metals and mapped by X-ray fluorescence on beamline ID15A. Credits: M. Strotton.