- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2016
- Electronic structure, magnetism and dynamics
- Advanced X-ray spectroscopic tool for actinide research
Advanced X-ray spectroscopic tool for actinide research
The work is the first illustration of the ability of high-energy-resolution fluorescence-detection X-ray absorption spectroscopy (HERFD-XAS) to directly probe the crystal-field splitting in the 5f shell of actinides and to resolve the charge-transfer satellites. This is a breakthrough for actinide science, allowing for the straightforward extraction of desired information from the spectroscopic method, which is also easy to interpret and easy to calculate.
For strongly electron-correlated systems, X-ray absorption spectroscopy (XAS) is viewed as a technique capable of providing information on the ground state character despite the presence of the core-hole in the final state of the spectroscopic process. In the 3d transition metal compounds, XAS at the metal 2p edge is routinely used to probe the magnitude of the crystalline electric field (CEF) splittings in the 3d shell and the degree of the 3d hybridisation in the ground state. However, for the 5f shell of actinides, such a usage of XAS is difficult. At the U 3d and 4d edges, where the transitions to the 5f states are probed, core-hole lifetime broadenings (full width at half maximum) are about 3.2 eV and 4.2 eV, respectively, while at the 5d edge, the broadening can be as large as 6.0 eV. Therefore, the conventional XAS spectra of actinide materials at the 3d and 4d edges often appear as single lines where splittings of the 5f states (e.g., due to the CEF interaction) are indistinguishable.
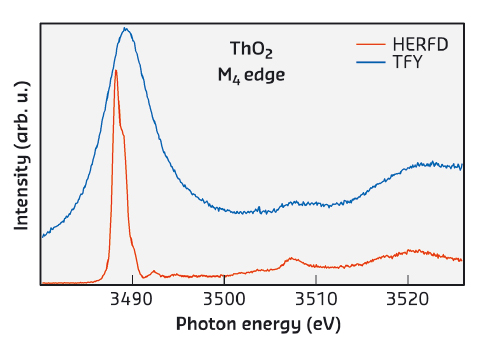
An advanced technique, HERFD-XAS, has been recently employed for measurements at the 3d edge of U compounds [1]. This has led to a tremendous improvement in the experimental resolution due to reduced core-hole lifetime broadening and revealed additional structures in the XAS spectra, not measurable before (see Figure 23). The effects of the hybridisation of the valence states of actinides with ligand states in compounds are expected to result in the appearance of charge-transfer satellites in the XAS spectra. The interpretation of such satellites can provide information on the degree of the 5f hybridisation, covalency of the compound, and the nature of the ground state.
 |
|
Fig. 23: Total fluorescence yield (TFY) and HERFD spectra at the Th M4 edge of ThO2. |
Here, we demonstrate, using ThO2, that the HERFD-XAS technique now allows us to directly probe the CEF splittings in the 5f shell with the results clearly shown in the spectra. An analysis of the newly-resolved charge-transfer satellites indicates significant occupancy of Th valence states which contradicts the common view of ThO2 as an ionic system (see e.g. [2]).
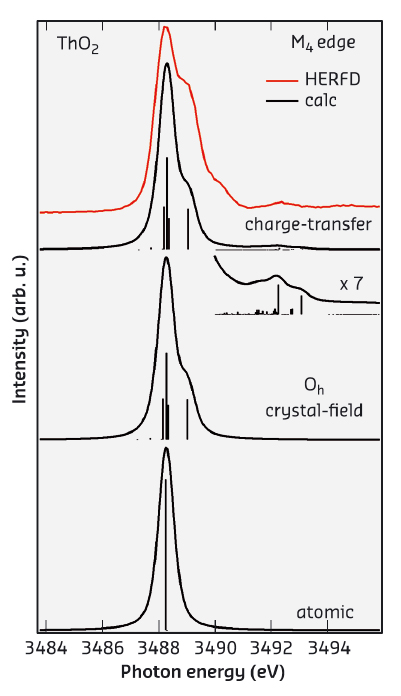
Figure 24 shows the HERFD-XAS spectrum at the Th M4 edge of ThO2 (recorded at beamline ID26) which is compared with the results of the Anderson impurity model (AIM) calculations taking into account both Th 5f-O 2p and Th 6d-O 2p charge-transfer. In particular, the structure at around 3492.5 eV appears in the calculated spectrum only upon inclusion of the Th 6d-O 2p charge transfer. Based on agreement with experiment, the AIM calculations for the ground state indicate that the occupancy of the Th 6d states (0.20 electrons) is larger than that of the Th 5f states (0.11 electrons). This result does not support an ionic character for ThO2.
 |
|
Fig. 24: Experimental and calculated XAS spectra at the Th M4 edge of ThO2. |
The improved resolution of the HERFD-XAS technique allows us to resolve the shoulder at ~0.8 eV above the main maximum of the Th M4 edge of ThO2. The origin of this shoulder becomes clear from a comparison of the experimental data with atomic and CEF multiplet theory for the Th(IV) ion in Figure 24. The atomic multiplet calculations for the transitions between the 3d105f0 and 3d95f1 configuration in the 1S0 ground state produce a single multiplet pole while putting the Th(IV) ion in the cubic (Oh) CEF environment reveals CEF split states of the 3d95f1 configuration thus producing the shoulder at ~0.8 eV above the main peak in the calculated spectrum for the Wybourne's CEF parameter values of B4=–1.30 eV and B6=0.55 eV. The improvement in resolution offered by HERFD-XAS now allows the CEF interactions to be probed for the 5f shell and to extract the information about the CEF effects directly from the XAS data in the manner commonly used for the L2,3-edges of 3d transition metal systems.
Principal publication and authors
High-resolution X-ray absorption spectroscopy as a probe of crystal-field and covalency effects in actinide compounds, S.M. Butorin (a), K.O. Kvashnina (b,c), J.R. Vegelius (a), D. Meyer (d) and D.K. Shuh (e), PNAS 113, 8093-8097 (2016); doi: 10.1073/pnas.1601741113.
(a) Uppsala University, Uppsala (Sweden)
(b) The European Synchrotron, Grenoble (France)
(c) HZDR, Institute of Resource Ecology, Dresden (Germany)
(d) CEA-ICSM, Marcoule (France)
(e) Lawrence Berkeley National Laboratory, Berkeley (USA)
References
[1] K.O. Kvashnina, S.M. Butorin, P. Martin and P. Glatzel, Phys. Rev. Lett. 111, 253002 (2013).
[2] The chemistry of the actinide and transactinide elements, L.R. Morss, N.M. Edelstein, J. Fuger (Eds.), Springer, Dordrecht, The Netherlands (2010).



