- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2016
- Electronic structure, magnetism and dynamics
- Decomposition of the hydrogen storage material Mg(BH4)2 followed by in situ X-ray Raman scattering spectroscopy
Decomposition of the hydrogen storage material Mg(BH4)2 followed by in situ X-ray Raman scattering spectroscopy
X-ray Raman scattering spectroscopy (XRS) combines the benefits of soft-X-ray spectroscopy and the properties of hard X-rays making it especially valuable for the in situ study of (amorphous) lightweight/low-Z materials. This technique was used to unveil the hydrogen release reactions of the hydrogen storage material Mg(BH4)2 for temperatures up to 500°C and pressures up to 4 bar.
Mg(BH4)2 is one of the most promising candidates for next generation hydrogen storage materials based on metal borohydrides owing to its large hydrogen content and moderate hydrogen release conditions. However, the decomposition of Mg(BH4)2 is not yet fully understood and various hydrogen release pathways have been suggested. Reversibility of the hydrogen release process is a prerequisite for application as energy storage materials. The occurrence of highly stable decomposition products such as amorphous boron or MgB12H12 hinder this reversibility and their identification is difficult.
To clarify the possible pathways and decomposition reactions, we used XRS spectroscopy, at beamline ID20, at the boron K-edge and the magnesium L2,3-edges. In comparison with spectra of reference samples such as MgB12H12, MgB2, MgH2, MgO, Mg, H3BO3, and boron, a fingerprint analysis of both edges yields detailed quantitative information about the reaction products during decomposition. Moreover, tomographic images of the samples enclosed in the reaction chamber were recorded to probe the structural changes of the sample on the macroscopic scale.
 |
|
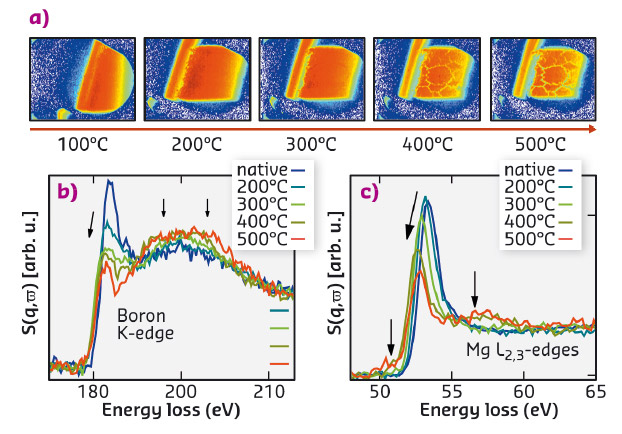
Fig. 12: a) Tomographic images of the Mg(BH4)2 sample enclosed in the reaction chamber for different annealing stages. b) XRS spectra of the boron K-edge and c) the magnesium L2,3-edge of Mg(BH4)2 measured during the thermally induced decomposition reaction. |
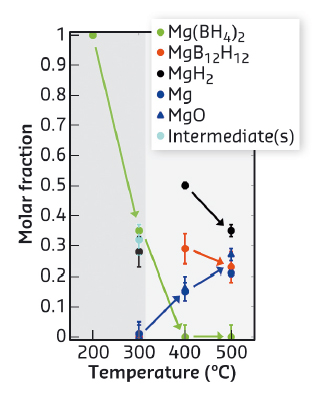
The structural integrity of the sample changed for temperatures just above 400°C as can be seen from macroscopic cracks which are due to significant volume changes during the formation of MgH, Mg, and MgO (see tomographic images in Figure 12a). The corresponding XRS spectra for temperatures as indicated are presented in Figure 12b and c for the boron K and the magnesium L2,3-edges, respectively. We observe clear spectral changes with increasing temperature accompanied by hydrogen release. Employing the fingerprint analysis utilising the reference spectra, we reveal the formation of decomposition intermediate(s) at 300°C. The results of this analysis are summarised in Figure 13. The intermediate(s) form with significant hydrogen release (grey shaded area in Figure 13). The unwanted phases MgB12H12 and (amorphous) boron are not observed in this reaction step. After annealing at 400°C, crystalline MgH2 and higher boranes (e.g. MgB12H12-like phases) are formed. These boranes decompose into the constituting elements magnesium and boron at higher temperatures up to 500°C. In a next step, detailed characterisation of the reaction intermediate(s) is foreseen via synthesis of possible intermediates to measure references and/or modelling of the intermediate's XRS spectra.
 |
|
Fig. 13: Quantitative analysis of the observed phases during the decomposition reaction of Mg(BH4)2. |
In this study, we were able to determine the major reaction products in the temperature driven decomposition of Mg(BH4)2. At 300°C a significant hydrogen release without formation of stable boron phases was observed which confirms the role of Mg(BH4)2 as a promising material for reversible energy storage, e.g. for mobile applications. XRS has proven to be a valuable tool to obtain quantitative information even on amorphous products during the hydrogen decomposition reaction and opens new perspectives for investigations, e.g. of nanoconfined samples, eutectic mixtures, and layer-protected hydrides.
Principal publication and authors
In situ characterization of the decomposition behavior of Mg(BH4)2 by X-ray Raman scattering spectroscopy, C.J. Sahle (a), S. Kujawski (b), A. Remhof (c), Y. Yan (d), N.P. Stadie (c), A. Al-Zein (a), M. Tolan (b), S. Huotari (d), M. Krisch (a) and C. Sternemann (b), Physical Chemistry Chemical Physics 18, 5397-5403 (2016); doi: 10.1039/C5CP06571B.
(a) ESRF
(b) Fakultät Physik / DELTA, Technische Universität Dortmund (Germany)
(c) Materials for Energy Conversion, Empa, Dübendorf (Switzerland)
(d) Department of Physics, University of Helsinki (Finland)



