- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2014
- Structure of materials
- Crystallographic architecture of a single zeolite ZSM-5 crystal
Crystallographic architecture of a single zeolite ZSM-5 crystal
Zeolites represent a very important class of natural and synthetic alumininosilicate materials with a wide range of applications including ion-exchange, adsorption and catalysis. The size and topology of zeolite cages and channels is determined by a highly crystalline 3-D porous network that consists of the Si-O tetrahedra. Substitution of Al for Si atoms in the crystalline structure leads to the formation of acidic sites that can catalyse a broad range of chemical reactions. This particular combination of Al-related acidic sites and shape-selective properties defined by their narrow pores (< 0.8 nm) makes zeolite catalysts a workhorse in numerous large-scale industrial processes.
Zeolite ZSM-5 is one of the most abundantly used solid acid catalysts in oil refining and petrochemical industries. To understand the catalytic performance of this material, large coffin-shape zeolite crystals (100 × 20 × 20 µm3) have frequently been investigated as model systems [1]. The crystals have been used in numerous micro-spectroscopic studies due to their well-defined structure and suitable size. They consist of at least 6 intergrown subunits (Figure 123) that may differ in their crystallographic orientation making the crystal properties highly anisotropic. Therefore one of the intriguing questions related to the structure and reactivity of zeolite ZSM-5 crystals is the precise orientation of the porous network in individual subunits and their impact on uptake and diffusion of guest molecules. This question has been mostly studied using a number of optical microscopy techniques providing only indirect evidence of the crystallographic orientation of the subunits.
 |
|
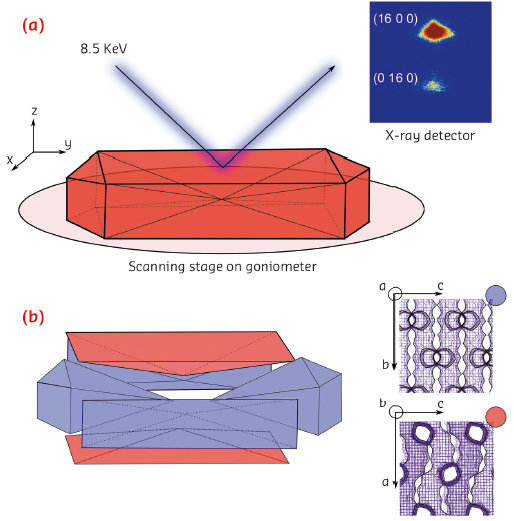
Fig. 123: a) Schematic of the µ-XRD imaging approach. b) Intergrowth structure of the zeolite ZSM-5 crystal and the crystallographic orientation of each subunit (colour-coded). |
Using highly focused X-rays at ID01, we conducted a micro-X-ray diffraction (µ-XRD) crystallographic imaging study of a single zeolite ZSM-5 crystal. Individual zeolite ZSM-5 crystals were mapped using an X-ray beam (8.5 keV) focused down to a size of 1 × 2 µm2. To minimise the footprint of the beam, we recorded higher order Bragg reflections in the range of 2θ > 70° (Figure 123a). The a and b lattice parameters of zeolite ZSM-5 are almost identical, which enables reliable identification of the crystalline phases based on distinct splitting of the higher order (16 0 0) and (0 16 0) reflections. Using those reflections, we further reconstructed diffraction intensity maps with lateral resolution of 2 µm and determined crystallographic orientation of the individual subunits. The results indicate 90° intergrowth structure of a zeolite ZSM-5 crystal, meaning that the top and bottom subunits have a 90° rotated crystal lattice as compared to the remaining subunits (Figure 123b). This implies that the direction of the straight and sinusoidal pores changes depending on the subunits, therefore having an impact on uneven uptake of guest molecules.
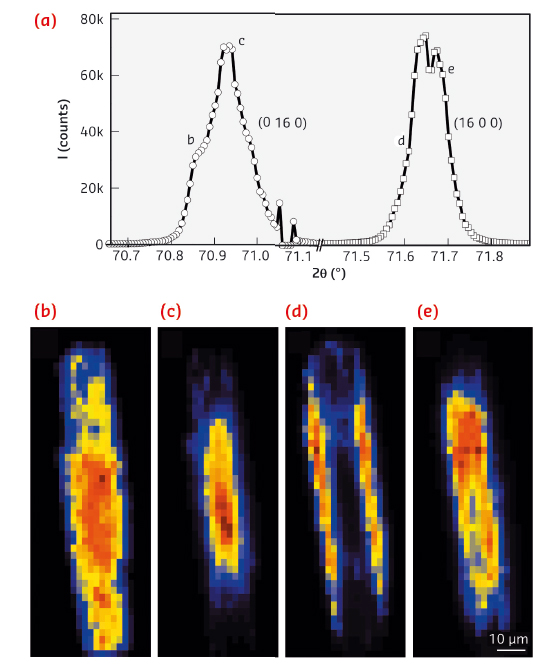
The high angular and spatial resolution of the technique also enables subtle changes in the lattice parameters to be resolved. It was possible to map the distortion of the crystal lattice spatially along the crystal x and y axes (Figure 124). The results point towards a very small, but detectable 0.02 Å expansion of the unit cell from the centre of the crystal towards the edges. This is explained by a macroscopic gradient in the concentration of Al atoms and consequently the heterogeneous distribution of acidic sites. As a result of this gradient, inner regions of the zeolite crystal are Si-rich, while Al atoms mostly concentrate in the outer crystalline layer near the surface – a phenomenon that to the best of our knowledge has never been crystallographically substantiated (Figure 124b).
 |
|
Fig. 124: a) Fine structure of the reconstructed single (x, y) pixel diffractograms, measured for the (16 0 0) and (0 16 0) Bragg reflections. b-e) Corresponding µ-XRD intensity maps of the Bragg peaks integrated for the 2θ regions as indicated in (a). |
With the method demonstrated here, it is possible to look at the crystalline structure not only of a single zeolite crystal – but also at any advanced crystalline material where structural and chemical gradients play an important role. In addition, hard X-ray diffraction provides an ideal tool to study chemical processes in situ, where one could benefit from the well-defined model systems, such as zeolite ZSM-5 crystals. Using a combination of µ-XRD and a wide range of micro-spectroscopic techniques, it is now possible to elucidate the structure-performance relationship for numerous processes that take place within functional materials.
Principal publication and authors
Z. Ristanovic (a), J.P. Hofmann (a), U. Deka (a), T.U. Schülli (b), M. Rohnke (c), A.M. Beale (a) and B.M. Weckhuysen (a), Angew. Chem. Int. Ed. 52, 13382 –13386 (2013).
(a) Inorganic Chemistry and Catalysis, Utrecht University (The Netherlands)
(b) ESRF
(c) Institute of Physical Chemistry, Justus-Liebig-University Giessen (Germany)
References
[1] L. Karwacki, M.H.F. Kox, D.A. Matthijs de Winter, M.R. Drury, J.D. Meeldijk, E. Stavitski, W. Schmidt, M. Mertens, P. Cubillas, N. John, et al., Nat. Mater. 8, 959–965 (2009).



