- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2012
- Structure of materials
- Charge order at the frontier between the molecular and solid states in Ba3NaRu2O9
Charge order at the frontier between the molecular and solid states in Ba3NaRu2O9
Strong many body correlations between electrons in solids result in extremely diverse properties including insulating antiferromagnetism, charge order and superconductivity. This is especially true in organic based materials like fullerides or the Κ-(BEDT-TTF)2X or (Pd[dmit]2)2 salts, due to the narrow electronic bandwidth resulting from the overlap of molecular orbitals [1]. Our recent investigation of Ba3NaRu2O9 shows that similar competition between ground states can also be observed in simple metal oxides which have quasi-molecular structures.
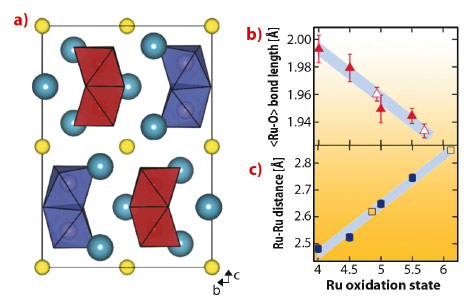
We synthesised powder samples of Ba3NaRu2O9 which were characterised by high resolution synchrotron X-ray diffraction measurements using ID31. The 6H hexagonal perovskite structure is found at room temperature (Figure 127a), with isolated Ru5.5+2O9 dimers of face-sharing octahedra packed into triangular layers. Previous reports indicate that a metal-insulator transition [2] and structural distortion occur below 210 K.
Below 210 K, we detected a monoclinic distortion and solved the structure in P2/c. The key feature of our structure is a splitting of the Ru5.5+2O9 dimers into two symmetry inequivalent units, implying charge ordering. Our refinements allowed us to examine distortions on the single-ion and molecular levels, and compare them to other members of the Ba3ARu2O9 family of known oxidation states. The valences of the two Ru sites are reflected [2] by a linearly varying <Ru-O> distance (Figure 127b), and the refined values for the P2/c structure are in agreement with ‘integer’ separation into 5+ and 6+ oxidation states. The inter dimer Ru-Ru distances also vary linearly with oxidation state (Figure 127c). This implies a well defined ‘bond order’ determined by the occupation of molecular orbital states and allows a precise estimate of the charge separation into 4.86(16)+ and 6.11(16)+ states.
 |
|
Fig. 127: a) Charge ordered P2/c structure of Ba3NaRu2O9 as determined from synchrotron powder X-ray diffraction projected down [100], sodium atoms are yellow spheres, barium atoms are blue. The symmetry inequivalent Ru2O9 dimers are shown in red (5+) and in purple (6+). b) The average Ru-O bond distance has a linear dependence on oxidation state in the Ba2ARu2O9 family of compounds with A = Na+, Ca2+, Nd3+ and Ce4+, which have similar ionic radii. The formal ruthenium oxidation states are 5.5+, 5+, 4.5+ and 4+, open symbols are from the P2/c phase. The shaded region shows the standard deviation of the calibration fit. c) A similar linear dependence upon formal oxidation state is found for the Ru-Ru distance. The symbols are as above. |
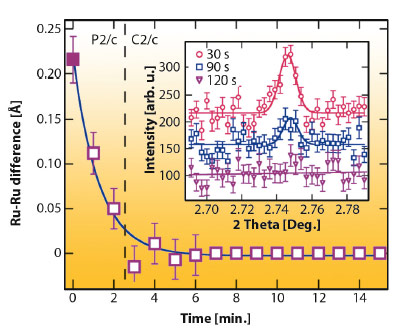
Although the P2/c phase of Ba3NaRu2O9 has large (~0.1 Å) atomic displacements, the charge order is unusually sensitive to external perturbations. We discovered that an X-ray induced melting of charge order is found below ~40 K. By cooling the sample to 10 K without the X-ray beam, then continuously measuring diffraction profiles, we found that the P2/c charge ordered structure transforms continuously to a higher symmetry C2/c structure in which only one Ru site is present (Figure 128). This phase was found to be stable for periods of hours at 10 K, and was not observed by neutron powder diffraction.
 |
|
Fig. 128: Difference between the refined Ru-Ru distance for the two dimer types in Ba3NaRu2O9 as a function of irradiation time at 10 K. The (h+k) = odd superstructure reflections (inset) also disappear, showing that the CO melts. |
Our LDA+U calculations, and analysis using an extended Hubbard model, show that it is the combination of local and molecular distortions with long-range Coulomb interactions that stabilise charge order in Ba3NaRu2O9. The low temperature X-ray induced melting of the charge order is possibly related to frustration of these degrees of freedom on the triangular lattice of dimers. We speculate that other strongly correlated phenomena await discovery in the broader class of hexagonal perovskite materials.
Principal publication and authors
S.A.J. Kimber (a,b), M. Senn (c), S. Fratini (d), H. Wu (e), A.H. Hill (a), P. Manuel (f), J.P. Attfield (c), D.N. Argyriou (a,g) and P.F. Henry (a,g), Phys. Rev. Lett. 108, 217205 (2012).
(a) ESRF
(b)Helmholtz-Zentrum Berlin für Materialien und Energie (Germany)
(c) Centre for Science at Extreme Conditions and School of Chemistry, University of Edinburgh (UK)
(d) Institut Néel-CNRS and Université Joseph Fourier, Grenoble (France)
(e) Laboratory for Computational Physical Sciences, Surface Physics Laboratory and Department of Physics, Fudan University (China)
(f) ISIS Science and Technology Facilities Council, Rutherford Appleton Laboratory (UK)
(g) European Spallation Source ESS AB, Lund (Sweden)
References
[1] B.J. Powell and R.H. McKenzie, J. Phys. Cond. Matt. 18, R827 (2006).
[2] K.E. Stitzer et al., J. Am. Chem. Soc. 124, 13877 (2002).



