- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2011
- Structural biology
- Development of a universal vaccine for Streptococcus agalactiae
Development of a universal vaccine for Streptococcus agalactiae
Several regions of a foreign protein antigen are the target of immune responses, however protective epitopes, the part of an antigen that is recognised by the immune system, are confined within a limited number of protein domains. Structural vaccinology involves the elucidation of domains embedding protective properties in order to design highly efficacious synthetic vaccines exclusively constituted by protective epitopes.
We have recently applied structural vaccinology to develop a universal anti-Streptococcus agalactiae (Group B Streptococcus (GBS)) vaccine. Like many other Gram-positive organisms, GBS features the pilus-like structures on its surface. These are known to play a crucial role in the interaction with the human host as they are involved in bacterial aggregation, adhesion to the host tissues and in biofilm formation.
GBS pili are constituted by three protein subunits, one forming the shaft of the organelle (also known as backbone protein (BP)), an ancillary protein (AP1) which decorates the pilus stem, and a second ancillary protein (AP2) often found at the base of the pilus and which has the role of anchoring the pilus to the cell wall. All three proteins are covalently linked to each other through a sortase-mediated transpeptidation reaction.
Sequence analysis of a large panel of GBS isolates revealed the existence of three pilus islands, PI-1, PI-2a and PI-2b, with all strains having at least one, and more frequently two, of the three islands. Although structurally similar, the BP subunit from one island shares relatively low homology with its counterparts in the other two islands. However, amino acid sequences of BP in strains carrying the same island are highly conserved, with the exception of the backbone subunit of PI-2a (BP-2a), which is grouped into six immunologically distinct variants.
GBS pili were discovered by serendipity while searching for protective antigens using reverse vaccinology. This and subsequent investigations demonstrated that AP1 and BP subunits elicit protective opsonophagocytic antibodies, with BP having the strongest immunogenic properties. From a vaccine standpoint, the fact that virtually all GBS isolates carry pili opens exciting perspectives for the design of universal pilus-based vaccines. However, the existence of eight immunologically different variants of the highly protective BP protein (one from PI-1, one from PI-2b and six from PI-2a) poses a severe challenge in vaccine manufacture.
 |
|
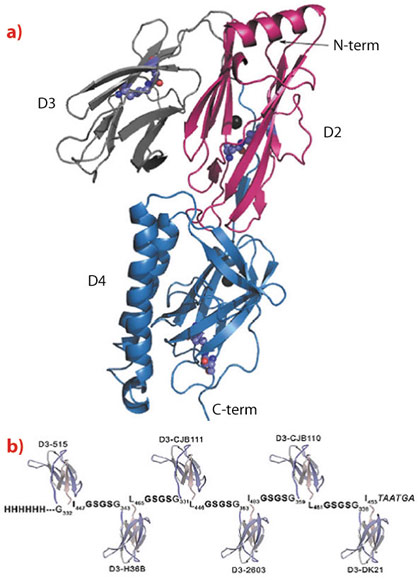
Fig. 109: a) Ribbon representation of the crystal structure of the BP subunit of GBS 515 strain carrying type 2a pili. The structure illustrates the four-domain organisation of the pilus protein. b) Schematic representation of the recombinant chimeric protein carrying the D3 domains of all six BP variants of type 2a pili. |
With the aim of simplifying the final vaccine formulation, the 3D structure of one of the six BP variants of PI-2a was resolved using diffraction data collected on beamline ID23-1. The structure revealed a four-domain organisation (D1 - D4, Figure 109a) with the 100 amino acid long D3 facing the external side of the pilus shaft. When each domain was independently expressed in E. coli and tested for protective activity only D3 induced high titers of opsonophagocytic antibodies protecting mice against a lethal challenge with GBS isolates expressing the homologous PI-2a pilus variant. When the 3D structure of the PI-2a BP variant was used to model the structures of the other five PI-2a BP variants, not surprisingly, it was found that all BPs share a similar four-domain structural organisation. In line with this observation, the D3 domains of the other variants also proved to be highly protective against homologous challenge.
Taking advantage of the limited size of D3, the six PI-2a D3 variants were fused in a single polypeptide chain (Figure 109b), which was efficiently expressed in E. coli. The recombinant chimera conferred strong protection in mice challenged with any of the GBS strains carrying PI-2a pilus variants.
This work paves the way for the development of a universal, broadly protecting GBS vaccine. GBS infections still represent a serious threat for newborns despite the intrapartum antibiotic prophylaxis of GBS-colonised pregnant women adopted by most industrialised countries. Since children delivered from women with high titers of anti-GBS opsonophagocytic antibodies are protected from GBS infections, vaccination of women of child bearing age and/or pregnant women appears to be the ideal long-lasting solution to infections of newborn babies. However, an effective anti-GBS vaccine is not yet available. One of the reasons for this is the existence of ten different GBS serotypes and therefore, for a vaccine to be broadly protective, antigens conserved in all serotypes must be identified. The fact that (i) all GBS isolates carry pili, (ii) their BP subunits induce protective antibodies, and (iii) the combination of three proteins (the PBs from PI-1 and PI-2b and the PI-2a chimeric protein) can potentially protect against all GBS isolates, offers a once-and-for-ever solution to the prophylaxis of this important human pathogen.
Principal publication and authors
A. Nuccitelli (a), R. Cozzi (a), L.J. Gourlay (b), D. Donnarumma (a), F. Necchi (a), N. Norais (a), J.L. Telford (a), R. Rappuoli (a), M. Bolognesi (b), D. Maione (a), G. Grandi (a) and C.D. Rinaudo (a), Proc. Natl. Acad. Sci. USA, 108, 10278-10283 (2011).
(a) Novartis Vaccines and Diagnostics, Siena (Italy)
(b) Department of Biomolecular Sciences and Biotechnology, and Centro Interdisciplinare Materiali e Interfacce Nanostrutturati, University of Milan (Italy)



