- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2011
- Structural biology
- A RAF-induced allosteric transition of KSR stimulates KSR and RAF phosphorylation of MEK
A RAF-induced allosteric transition of KSR stimulates KSR and RAF phosphorylation of MEK
The RAS-RAF-MEK-ERK signalling pathway relays extracellular stimuli to changes in cellular function and gene expression. Aberrant activation of this pathway through oncogenic mutations is responsible for a large proportion of human cancers. KSR (kinase suppressor of RAS) functions as an essential scaffolding protein to coordinate the assembly of the protein kinases RAF, MEK and ERK into dynamic signalling complexes [1,2]. Such complexes facilitate the sequential phosphorylation of MEK by RAF and then ERK by MEK. Although the role of KSR as a scaffolding molecule to coordinate the assembly of RAF, MEK and ERK is well established, how KSR promotes stimulatory RAF phosphorylation of MEK is less well defined. Moreover, there has been significant debate as to whether KSR is an authentic protein kinase or, instead, represents a pseudo-kinase, that is, a protein with structural similarity to a protein kinase, but without catalytic activity. To address these questions we determined the crystal structure of a complex of the kinase domain of KSR2 (KSR2KD) with MEK.
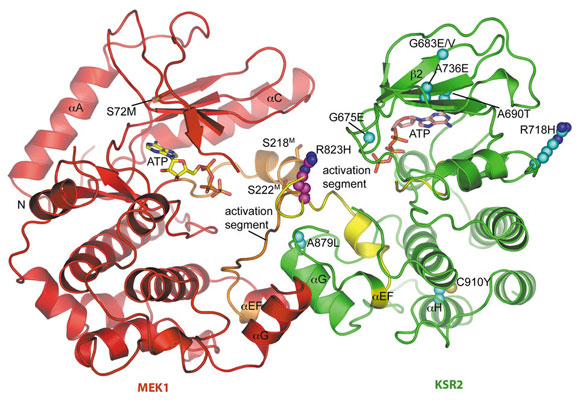
KSR2KD and MEK1 dimerise, and the crystal structure of the KSR2KD:MEK1 dimer was determined using data collected at beamline ID14-4. KSR2KD:MEK1 assemble into a tetramer through a KSR2KD homodimer interface centred on Arg718K of KSR2 KD (superscripts ‘K’ and ‘M’ refer to KSR2 and MEK1, respectively). KSR2KD and MEK1 molecules interact with their catalytic sites facing each other through their activation segments and αG helices (Figure 106). MEK’s activation segment, incorporating RAF phosphorylation sites Ser218M and Ser222M, comprises a short α-helix connected to a segment of extended chain that forms an anti-parallel β-sheet with the KSR2 activation segment (Figure 106). The integrity of the complex is likely conferred by the more extensive interface created by engagement of their respective αG helices.
 |
|
Fig. 106: Overall view of the KSR2KD:MEK1 heterodimer showing the face-to-face configuration of KSR2 (green) and MEK1 (red). Activation segments of KSR2 and MEK1 shown in yellow and orange, respectively. KSR loss of function mutations are shown with C-atoms as cyan spheres. |
The KSR2KD:MEK1 crystal structure indicated that KSR has the potential for catalytic activity possibly necessary for KSR function. Electron density maps reveal well-defined density for ATP-Mg2+ at the KSR2KD catalytic site. Although Asp803K of the DFG motif is shifted slightly out of position, the metal coordinating Asn791K and the general base Asp786K of the catalytic loop adopt conformations typical of conventional protein kinases. However, due to the position of its αC helix, KSR2KD adopts an inactive conformation in this crystal structure.
To examine putative KSR2 catalytic function we conducted in vitro kinase assays using an analogue-specific (as1) mutant of KSR2 incubated with the modified ATP analogue (A*TPγS) and found clear evidence for MEK phosphorylation. These data, together with results using a range of kinase inhibitors with wild type KSR2, demonstrated that KSR is an authentic protein kinase.
KSR assembles MEK-KSR-RAF ternary complexes responsible for promoting RAF phosphorylation of MEK. A model for how KSR and RAF interact through a KSR-RAF heterodimer interface was proposed based on similarities between the KSR homodimer interface and the BRAF homodimer interface. Both KSR and RAF homo-dimer interfaces are centred on an equivalent and conserved residue (Arg718 in KSR), therefore we proposed that KSR and RAF dimerise through the same interface. Formation of a KSR2:BRAF heterodimer would be accompanied by a shift of the KSR2 αC helix to an active conformation. A BRAF-induced conformational shift of the KSR2 αC helix into the active position would enhance KSR2 kinase activity and influence its interaction with the activation segment of MEK, thereby affecting RAF’s capacity to phosphorylate MEK. Consistent with this notion, RAF was found to allosterically stimulate KSR kinase activity. Moreover, the change of KSR tertiary structure modulates the structure of the MEK activation segment, facilitating its phosphorylation by RAF in trans.
This work reveals KSR as an effector molecule, receiving a stimulatory signal from an activated regulatory RAF molecule and relaying this to modulate the accessibility of the MEK activation segment for phosphorylation by catalytic RAF.
Principal publication and authors
D.F. Brennan (a), A.C. Dar (b), N.T. Hertz (b), W.C. Chao (a), A.L. Burlingame (c), K.M. Shokat (b) and D. Barford (a), Nature 472, 366-9 (2011).
(a) Division of Structural Biology, Institute of Cancer Research, Chester Beatty Laboratories, London (UK)
(b) Howard Hughes Medical Institute and Department of Cellular and Molecular Pharmacology, University of California San Francisco (USA)
(c) Department of Pharmaceutical Chemistry, University of California San Francisco (USA)
References
[1] A. Claperon and M. Therrien, Oncogene 26, 3143-58 (2007).
[2] W. Kolch, Nat Rev Mol Cell Biol 6, 827-37 (2005)



