- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2011
- Electronic structure and magnetism
- Visualising clean catalytic technologies in action
Visualising clean catalytic technologies in action
Catalysis is a rich, multidisciplinary field with a global socio-economic impact ranging from improved air quality to the design of new HIV therapies, and creation of bulletproof fabrics. Heterogeneous catalysis is currently experiencing a global renaissance, with interest soaring in new predictive quantum chemical models and experimental synthetic and analytical methodologies able to deliver tailored catalyst formulations for diverse chemistries. However, the rational design of new heterogeneous catalysts for sustainable chemical technologies requires molecular level insight into surface chemistry, and nanoengineering approaches to achieve precise control over the structure and reactivity of novel functional materials. Critical to this success is correct identification of the active centre participating in the catalytic cycle, and recognition that such active sites are responsive to changing reaction environments. Aerobic selective oxidation (selox) of alcohols offers an environmentally-benign synthetic route to key chemical intermediates used across the fine and agrochemical industries, notably in flavouring and fragrance preparation [1]. Despite their commercial importance, the nature of the active species within palladium catalysts used to drive these transformations has remained shrouded in mystery.
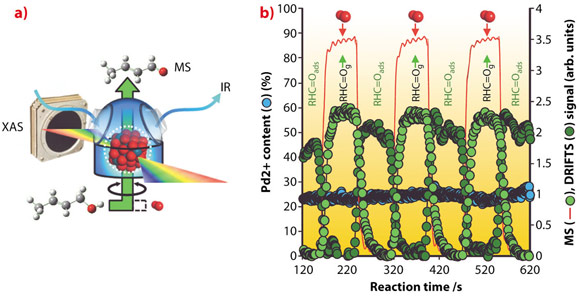
Operando techniques (wherein measurements are made under realistic reaction conditions mimicking those of practical catalytic processes) provide a powerful means to visualise catalysts in action, and derive critical mechanistic insight impossible to obtain by conventional means. We therefore utilised an experiment developed on ID24, in which dispersive EXAFS, diffuse-reflectance IR and mass spectrometry are coupled in a synchronous fashion [2], to enable time-resolved studies of vapour phase, allylic alcohol selective oxidation over Pd nanoparticles (Figure 87).
 |
|
Fig. 87: a) Schematic of operando catalyst cell. b) Time-resolved, synchronous DRIFTS/EDE/MS results showing crotonaldehyde formation, and desorption, under alternating O2/crotyl alcohol pulses over partially oxidised Pd nanocatalysts. |
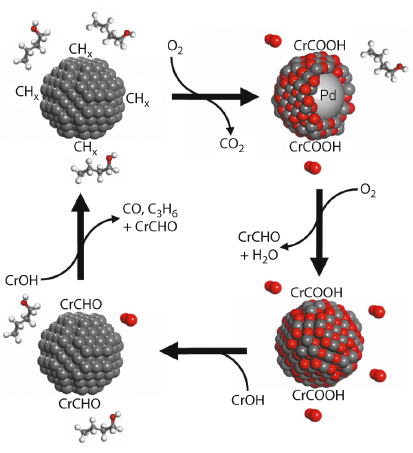
By carefully pulsing either alcohol or oxygen reactants over our catalysts, we were able to simultaneously determine the palladium oxidation state, and quantify the surface and gas phase concentrations of the desired, reactively-formed aldehyde product, along with undesired secondary products such as propene, CO and CO2. Contrary to the popular belief of the last century’s published literature, our operando measurements have conclusively revealed that partially oxidised palladium surfaces, and not palladium metal, are active towards selective oxidation, with in situ catalyst reduction under alcohol-rich environments responsible for undesired decarbonylation and combustion chemistry (Figure 88). Such insight is helping to direct the design of next-generation, tunable nanocrystalline and nanoporous clean technologies for the aerobic selox of diverse organic molecules.
 |
|
Fig. 88: Reaction-induced surface restructuring of Pd nanocatalysts elucidated during aerobic selective oxidation of crotyl alcohol to crotonaldehyde. |
Principal publication and authors
A.F. Lee (a), C.V. Ellis (a), J.N. Naughton (b), M.A. Newton (c), C.M.A. Parlett (a) and K. Wilson (a), J. Am. Chem. Soc. 133, 5724–5727 (2011).
(a) Cardiff Catalysis Institute, School of Chemistry, Cardiff University (UK)
(b) Department of Physics, University of York, York (UK)
(c) ESRF
References
[1] C.P. Vinod, K. Wilson and A.F. Lee, J. Chem. Technol. Biotechnol. 86, 161-171(2011).
[2] M.A. Newton, C. Belver-Coldeira, A. Martinez-Arias and M. Fernandez-Garcia, Nat. Mater. 6, 528-532 (2007).



