- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2009
- Structural biology
- Fluorescence of plant light-harvesting complex II in single crystals
Fluorescence of plant light-harvesting complex II in single crystals
Most of the solar power utilised in photosynthesis is collected and transmitted to the energy-converting reaction centres by peripheral light-harvesting complexes. Among these, the chorophyll a/b light-harvesting complex of photosystem II (LHC-II) is the major antenna complex in plants, accounting for roughly half of the total chlorophyll content in chloroplasts, and thus for most of the green matter in the biosphere. Recently, the role of LHC-II in energy-dependent non-photochemical quenching (qE) has come into focus. qE is a photoprotective mechanism used by plants to dissipate surplus excitation energy safely when the photochemical capacity of the photosystem is exceeded in strong light. Of the two X-ray structures of LHC-II in the protein data bank, the one at higher resolution (2.5 Å, pdb code 2BHW) was determined from data collected at the beamline ID14-1 with crystals of the pea complex [1]. The other structure at 2.7 Å resolution is that of the spinach complex (pdb code 1RWT). With these two structures in hand, the search was on for the exact position of the quenching centre in the complex, and the molecular mechanism of quenching. A prominent model for the quenching process proposes that a conformational change switches LHC-II from its active, energy-transmitting state to an inactive, quenched state in which excitation energy is dissipated in the form of heat.
For a better understanding of the quenching mechanism, it was therefore important to know which of these two hypothetical states of the LHC-II was represented by the crystal structures. An earlier spectroscopic study [2] had concluded from an apparent red-shift and the shorter fluorescence lifetime of LHC-II crystals that the spinach structure showed the quenched state. Despite the large differences in crystallisation conditions and crystal forms, the two crystal structures are virtually identical, this would mean that the pea complex is in the same quenched state. We decided to investigate this further by analysing the fluorescence properties of LHC-II in single crystals at the ESRF Cryobench laboratory.
 |
|
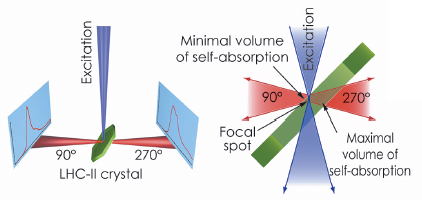
Fig. 123: The optical system of the Cryobench used to record fluorescence spectra of LHC-II single crystals in two different detection geometries, corresponding to front-side (at 90°) and back-side (at 270°) detection with respect to the crystal and the exciting light beam. |
The possibility of examining individual single crystals mounted in cryo-loops, as opposed to a suspension of crystals used in the previous studies, and to align the crystals precisely relative to the incident light beam were decisive for the success of our study. We demonstrated that fluorescence emission spectra of single LHC-II crystals are critically dependent on crystal orientation (Figure 123). In certain orientations, the low-temperature emission spectrum shows strong fluorescence emission at 680 nm. This is characteristic of the active, energy-transmitting complex and had previously been observed only with LHC-II in detergent solution. In other crystal orientations, in which the emitted photons travel through the thickness of the crystal before arriving at the recording device, a broad 700 nm band predominates, demonstrating that the apparent red-shift of LHC-II fluorescence in single crystals is due to self-absorption. These observations prove that both crystal structures of LHC-II show the complex in an active, energy-transmitting state.
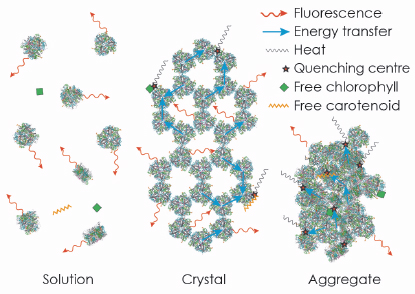
We then extended our study to provide further evidence for our conclusion by an extensive analysis of the fluorescence lifetime of LHC-II in single crystals. For reference, we performed a parallel analysis of LHC-II fluorescence lifetimes in detergent solution and aggregates, commonly thought to represent the active and quenched state of the complex, respectively. Our results indicated that the average fluorescence lifetime of LHC-II in single crystals at 100 K, although much shorter than in solution, is significantly longer than in aggregates, indicating a smaller extent of quenching. The time-resolved data also revealed that a substantial fraction of the LHC-II trimers in the crystals retains the long fluorescence lifetime of the complex in solution, so that the observed shorter fluorescence lifetime must occur due to migration of the excitation energy to quenching centres randomly distributed within the crystal (Figure 124). We conclude that quenching of excitation energy in LHC-II is due to the molecular interaction with external pigments in vitro or other pigment-protein complexes in vivo, and does not require a conformational change within the complex.
 |
|
Fig. 124: The strong fluorescence of LHC-II in detergent solution is quenched in crystals and aggregates due to random interactions of trimers, or of trimers with free pigments. |
References
[1] J. Standfuss, A.C. Terwisscha van Scheltinga, M. Lamborghini and W. Kühlbrandt, Embo J 24, 919 (2005).
[2] A.A. Pascal, Z. Liu, K. Broess, B. van Oort, H. van Amerongen, C. Wang, P. Horton, B. Robert, W. Chang and A. Ruban, Nature 436, 134 (2005).
Principal publication and authors
T. Barros (a), A. Royant (b,c), J. Standfuss (a), A. Dreuw (d) and W. Kühlbrandt (a), EMBO Journal 28, 298 (2009).
(a) Department of Structural Biology, Max Plank Institute of Biophysics, Frankfurt am Main (Germany)
(b) Institut de Biologie Structurale Jean-Pierre Ebel (France)
(c) ESRF
(d) Institute for Physical and Theoretical Chemistry, Johann Wolfgang Goethe-University Frankfurt (Germany)



