- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2001
- Soft Condensed Matter
- A Mineral Liquid-Crystalline Lamellar Phase
A Mineral Liquid-Crystalline Lamellar Phase
Nanometre-scale ordering is well known to appear spontaneously when anisotropic organic moieties form liquid-crystalline phases. Though much less common, this behaviour is also observed for suspensions of anisotropic mineral nanoparticles and its study is currently a challenging and active research area in materials science [1-3]. In this context, we have recently discovered that the low-dimensional layered solid state compound H3Sb3P2O14 can be exfoliated in water to yield homogeneous, transparent suspensions of extended covalent sheets. Moreover, we found that these suspensions form a liquid-crystalline lamellar phase in a wide range of volume fractions (Figure 51). In this phase, the mineral extended sheets are parallel and regularly stacked. In a small-angle X-ray scattering experiment on the ID2 beamline, a series of sharp peaks are observed and they can be indexed to the (00l) reflections of a lamellar structure (Figure 52). Experiments on very dilute suspensions show that the form factor of the particles is the one of 2-dimensional (2-D) planar objects of at least 300 nm diameter. Wide-angle X-ray diffraction experiments show the existence of fairly thin diffraction lines which prove that the covalent sheets keep their (2-D) long-range atomic positional order even at high dilution (Figure 52 inset). Therefore, the mineral lamellar phase is very different from its organic counterparts that are usually comprised of liquid layers at such dilutions.
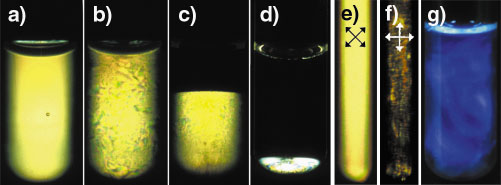 |
Fig. 51: Test tubes observed between crossed polarisers (a-f), the isotropic phase in (c) and (d) appears dark: (a) Lamellar gel phase ( |
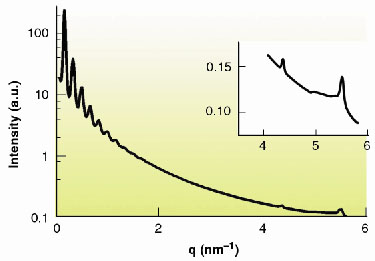 |
Fig. 52: SAXS intensity curve of "powder" samples versus scattering vector modulus q, showing reflections up to the tenth order due to the lamellar period. Inset: Thin diffraction lines observed at wide angles. |
The lamellar period d increases as the volume fraction decreases, i.e. as more water is inserted between the mineral sheets. The lamellar phase can actually be swollen to a very large extent. It remains stable down to ~0.75 % where the lamellar period reaches 225 nm, to be compared with the covalent sheet thickness of only 1 nm. Thus, d can be continuously tuned from 1.5 to 225 nm. When
< 0.75 %, the suspensions are biphasic with a clear interface between the birefringent lamellar phase and an isotropic phase. This behaviour is indeed expected for the swelling of a lamellar phase: once the maximum swelling is reached, water molecules can no longer be inserted into the interlamellar space and excess water is expelled, leading to phase separation. Adding salt to the system leads to a decrease of the maximum lamellar period and to flocculation, which strongly suggests that electrostatic interactions are responsible for the thermodynamic stability of the liquid crystal.
This mineral lamellar phase can be very well oriented by mechanical shearing in a Couette cell mounted on the beamline, resulting in a very anisotropic scattering pattern (Figure 53). The mineral sheets are then aligned parallel to the shearing surfaces, as expected intuitively. This strong alignment was induced even at a very low shear rate and does not relax when the shearing is stopped. The lamellar phase can also be aligned by applying a strong magnetic field as the mineral sheets then orient their normal parallel to the magnetic field. This property can be used to induce the partial alignment of dissolved biomolecules. This phase, which has neither 13C nor 1H when prepared in D2O, seems particularly promising for the structural determination by liquid-state NMR of non-labelled biomolecules.
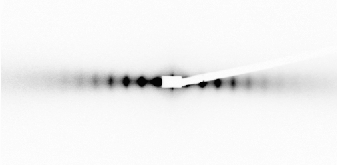 |
Fig. 53: SAXS 2-D scattering pattern of a sample sheared in a Couette cell, in the "tangential" geometry (d = 175 nm). |
References
[1] For a review, see: J.-C.P. Gabriel and P. Davidson, Adv. Mat., 12, 9 (2000).
[2] A.B.D. Brown, C. Ferrero, T. Narayanan and A.R. Rennie, Eur. Phys. J. B, 11, 481 (1999).
[3] F.M. van der Kooij, K. Kassapidou and H.N.W. Lekkerkerker, Nature, 406, 868 (2000).
Principal Publication and Authors
J.-C.P. Gabriel (a), F. Camerel (a), B.J. Lemaire (b), H. Desvaux (c), P. Davidson (b) and P. Batail (a), Nature, 413, 504 (2001).
(a) FRE 2068 CNRS, Nantes (France)
(b) UMR 8502 CNRS, Orsay (France)
(c) URA 331 CEA/CNRS, Saclay (France)



