- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2001
- Soft Condensed Matter
- Structure of Live Muscle
Structure of Live Muscle
Muscle tissues are fibrous assemblies, principally actin and myosin filaments, which have the important function of converting chemical energy into force and/or directed motion. Given that the structural organisation of the filaments is relatively well ordered, it is possible to obtain X-ray diffraction diagrams that extend to resolutions well into the molecular level. Studies using synchrotron radiation allow non-invasive probing of motions of the muscle proteins at a molecular level whilst the live muscle undergoes various forms of contraction. However, there are numerous technical problems associated with high-resolution X-ray diffraction studies of muscle tissues: the diffracting power of muscle tissues is weak; the angular resolution required is that needed to resolve unit cell sizes of at least 2500 nm; the time available to capture information about important structural intermediates is milliseconds or less. To overcome these difficulties, the use of synchrotron radiation sources, with their unusually high brilliance, has been mandatory for many years [1]. With the development of third generation, high-brilliance, high-energy synchrotron radiation sources, such as the ESRF, it has been technically possible to fully resolve the splitting in the diffraction diagrams of muscle tissues undergoing contraction [2]. The work described here was carried out on beamline ID2.
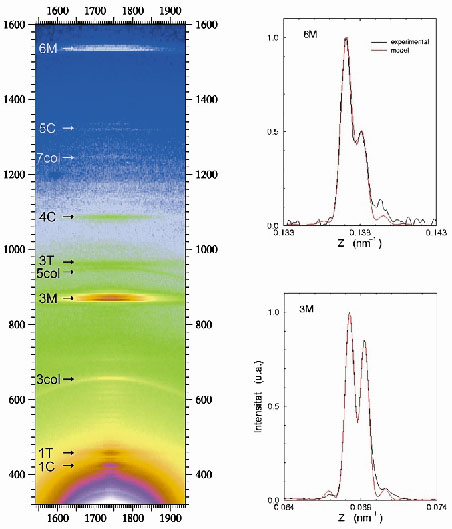 |
Fig. 47: (Left) Portion of the X-ray diffraction diagram yielded by a muscle at the peak of isometric contraction. This shows the third (3M) and sixth (6M) meridional orders, which are due to the axial disposition of the myosin heads protruding from the thick filament backbone. Other labels point to reflections arising from the C-protein (C), troponin (T) and collagen (col). (Right) Highly expanded trace (black lines) of the axial intensity distribution showing the interference induced splits in the 3M (bottom) and 6M (top) meridional orders. The red line is a fit to the experimental data from which the phase information is derived. |
Thick myosin filaments adopt a polar disposition on either side of the so-called M-lines, which bisect the muscle cell. The myosin diffraction units thus disposed produce X-ray interference phenomena (Figure 47) in a manner similar to the classical two-slit interference effects. This can, of course, provide phase information from which electron density maps of the axial disposition of the myosin heads can be constructed giving direct structure information about the disposition of the myosin heads during contraction. Resolution and measurement of the relative intensities of the peaks in each cluster constituting the meridional reflections shows that during isometric contraction each myosin head in a crown pair has a distinct structural disposition. Given the structure derived (Figure 48), it must be concluded that only one of the heads can stereo-specifically interact with the thin actin filament at any one time. This leads to the need to revise many of the assumptions usually made in the interpretation of X-ray diffraction data, as well as opening up the possibility to establish model-independent structural information in other forms of contraction. Recent results (unpublished) have also included the determination of the structural behaviour of the myosin heads during the transition from isometric to isotonic contraction.
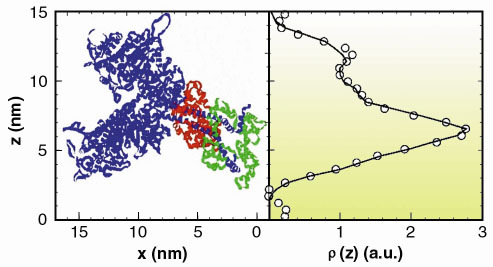 |
Fig. 48: Experimentally determined electron density distribution corresponding to the axial mass projection of the pair of myosin heads in a crown (circles). The line is the derived electron density distribution from the structure shown in the left panel [3]. Note that in the muscle the myosin filament backbone runs vertically on the right hand side of this representation; the double helix formed by the actin filament would run vertically on the left hand side. |
References
[1] H.E. Huxley, A.R. Faruqi, J. Bordas, M.H.J. Koch and J.R. Milch, Nature, 284, 140 (1980).
[2] J. Bordas, J. Lowy, A. Svensson, J.E. Harries, G.P. Diakun, J. Gandy, C. Miles, G.R. Mant and E. Towns-Andrews, Biophys. J., 66, 99 (1995).
[3] I. Rayment, W.R. Rypniewski, K. Schmidt-Base, R. Smith, D.R. Tomchick, M.M. Benning, D.A. Winkelmann, G. Wesenberg, and H.M. Holden, Science 261, 50-57 (1993).
Principal Publication and Authors
J. Juanhuix (a), J. Bordas (a), J. Campmany (a), A. Svensson (b), M.L. Bassford (b) and T. Narayanan (c), Biophys. J. 80, 1429 (2001).
(a) Laboratori de Llum Sincrotro, Campus Universitat Autonoma de Barcelona, Bellaterra (Spain)
(b) Department of Physics and Astronomy, Leicester University (UK)
(c) ESRF



