- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2012
- Structure of materials
- A surface science approach in aqueous phase used to rationalise the preparation of heterogeneous catalysts
A surface science approach in aqueous phase used to rationalise the preparation of heterogeneous catalysts
The rational design of heterogeneous catalysts involves the implementation of model approaches, “surface science” being the best known. This approach aims to model a complex industrial catalyst using simple chemical systems most often presented in the form of plane monocrystalline supports capable of reproducing, at least in part, the physicochemical behaviour of a metal particle or a pulverulent metal oxide with a large specific surface area.
This study focused on catalysts using Ni on alumina (Ni/Al2O3), which can be found in many applications such as steam reforming (hydrogen production from hydrocarbons), hydrogenation and hydrotreating (removal of S, N, O and metals from petroleum fractions). To model the industrial γ-alumina oxide support (poorly crystalline and exhibiting many different crystallographic faces) we decided to use single crystal wafers of α-alumina in different crystallographic orientations to model the different surface groups (hydroxyl) of the industrial support.
The originality of this study lies in how the catalyst was synthesised. This was carried out in the aqueous phase (as done industrially) and the characterisation was carried out under ambient conditions. This is in contrast to more traditional surface science approaches, where the model catalyst is synthesised under ultra-high vacuum, industrially far less realistic.
The use of oriented single crystals required a characterisation technique capable of providing molecular information for very low concentrations of active phase (Ni(II) in this case) deposited on the oxide surface. Grazing incidence EXAFS spectroscopy (grazing-incidence XAS or GI-XAS) turned out to be an ideal technique for such a study. This technique has been implemented on several complementary beamlines during this study: BM30B (FAME) and BM08 (GILDA) at the ESRF and DIFFABS and SAMBA at SOLEIL.
The use of oriented monocrystals coupled with the polarisation of the synchrotron beam yielded new information about the orientation of the supported active phase and showed that the crystalline orientation of the oxide support strongly governed speciation (chemical distribution) of the active phase. When Ni(II) was adsorbed in the aqueous phase, Ni K-edge EXAFS revealed an oriented precipitation of nickel hydroxide (Ni(OH)2) on the (1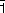 02) surface of α-alumina. For example, Figure 137 shows the effect of polarisation on the second peak in the Fourier transform of the EXAFS signal (Ni-Ni distances): this peak is very pronounced for a single crystal orientation parallel to the electric field vector,
02) surface of α-alumina. For example, Figure 137 shows the effect of polarisation on the second peak in the Fourier transform of the EXAFS signal (Ni-Ni distances): this peak is very pronounced for a single crystal orientation parallel to the electric field vector, 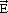 , whereas the intensity of the same Ni-Ni peak decreases sharply when the sample is oriented perpendicular to the electric field vector
, whereas the intensity of the same Ni-Ni peak decreases sharply when the sample is oriented perpendicular to the electric field vector 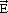 . In contrast, for the (0001) surface of α-alumina, no Ni deposit was observed, which shows the importance of the type of surface group exposed by the oxide for controlling the adsorption of the active phase.
. In contrast, for the (0001) surface of α-alumina, no Ni deposit was observed, which shows the importance of the type of surface group exposed by the oxide for controlling the adsorption of the active phase.
These results demonstrate at the molecular level that the oxide support does not merely act as a physical container of the active phase and that the nature of specific surface sites plays a key role in the formation and orientation of the nickel hydroxide precipitate. The fact that Ni(OH)2 precipitated only on the (1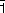 02) face can be explained by the minimisation of surface energy between the alumina and the nickel hydroxide.
02) face can be explained by the minimisation of surface energy between the alumina and the nickel hydroxide.
The use of a model system shows that the Ni dispersion on individual oxide particles is highly heterogeneous for an industrial catalyst as it will depend on the type of face exposed and therefore on the morphology of the oxide support. These findings also highlight the fact that each face of the alumina has a specific reactivity depending on which OH groups are exposed. Controlling the morphology of γ-alumina is therefore a key factor at the industrial scale in order to be able to control the deposition and dispersion of the active phase.
Principal publication and authors
A. Tougerti (a), I. Llorens (b,e), F. D’Acapito (c), E. Fonda (d), J-L. Hazemann (e,f), Y. Joly (f), D. Thiaudière (d), M. Che (a) and X. Carrier (a), Angewandte Chemie, International Edition 51, 7697-7701 (2012).
(a) UPMC-Université Pierre et Marie Curie, Laboratoire de Réactivité de Surface, Paris (France)
(b) CEA/DSM/INAC/CNRS, Grenoble (France)
(c) ESRF-GILDA, Grenoble (France)
(d) Synchrotron Soleil, Gif-sur-Yvette (France)
(e) ESRF-FAME, Grenoble (France)
(f) Institut Néel, CNRS-Université J. Fourier Grenoble (France)
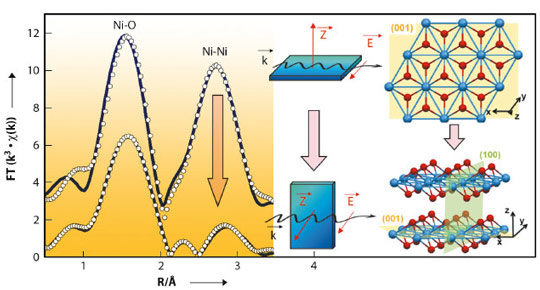
 02) surface of α-alumina. The solid blue line shows the experimental spectrum and the circle, refinement of the signal. The structure of the nickel hydroxide (Ni(OH)2) is shown on the right as seen from the top or on the side with respect to the (001) basal plane.
02) surface of α-alumina. The solid blue line shows the experimental spectrum and the circle, refinement of the signal. The structure of the nickel hydroxide (Ni(OH)2) is shown on the right as seen from the top or on the side with respect to the (001) basal plane.


