- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2009
- Soft condensed matter
- Structure of casein micelles and their complexation with tannins
Structure of casein micelles and their complexation with tannins
Micellar complexes of natively unfolded casein molecules constitute the major protein component of bovine milk. Casein micelles fluidise different casein molecules and solubilise calcium phosphate well above its solubility limit. The structure of the casein micelles has been the subject of extensive studies over the past decades but the details on the molecular level remain elusive [1]. While the internal structure of the casein micelles is a subject of debate, it has been widely accepted that the stability of the micelles is provided by an outer hairy layer of k-caseins and the calcium phosphate is contained in the form of colloidal nanoparticles in the core of micelles [1].
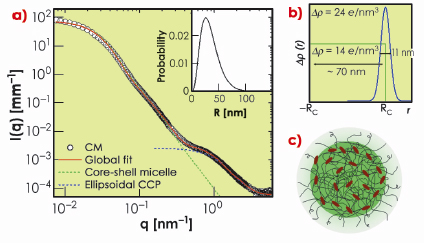
In this work, we have exploited the recent improvements in detection capability of SAXS [2] to elucidate the weak structural features of casein micelles otherwise submerged beneath the buffer scattering. Data collected over a wide scattering vector (q) range allowed us to reveal multiple structural levels. Figure 65a shows typical SAXS intensities from a casein micelle suspension after buffer subtraction. The data spanning three orders of magnitude in q can be modelled in terms of a polydisperse core-shell morphology with an internal structure consisting of ellipsoidal nanoparticles. The best fit electron density profile of the core-shell structure is shown in Figure 65b with a mean core radius (RC) of about 35 nm, polydispersity of 0.49, and shell thickness of 11 nm. The average size of micelles varies depending on their origin but the overall scattering features remain similar. The outer diffuse shell is primarily attributed to the ![]() -casein layer stabilising the large micelles. The excess electron density at the outer region of the protein core may be arising from the colloidal calcium phosphate (CCP). The best fit model for CCP corresponds to an ellipsoid scattering function with minor and major radii 2.5 nm and 0.71 nm, respectively. The resulting structure is schematically depicted in Figure 65c.
-casein layer stabilising the large micelles. The excess electron density at the outer region of the protein core may be arising from the colloidal calcium phosphate (CCP). The best fit model for CCP corresponds to an ellipsoid scattering function with minor and major radii 2.5 nm and 0.71 nm, respectively. The resulting structure is schematically depicted in Figure 65c.
 |
|
Fig. 65: a) Typical SAXS intensities of a casein micelle suspension of volume fraction ª 0.01. Continuous line represents global fit corresponding to a globular core–shell morphology and an internal structure consisting of colloidal calcium phosphate (CCP) particles reticulated in a protein matrix. The dotted lines indicate the individual globular and internal structural levels. The inset shows the size distribution of the globular micelles. b) Best fit electron density profile of core-shell structure. c) Schematic representation of a casein micelle with core-shell morphology and internal structure with decorated CCP. |
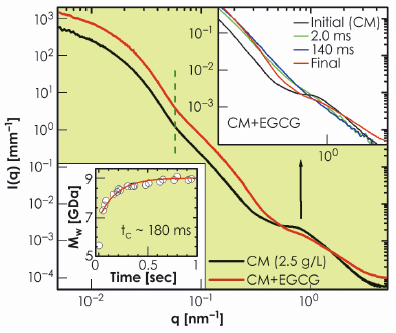
To validate this picture, we used small tannins (EGCG) which are polyphenols with a strong affinity towards proline residues on the caseins [3]. Figure 66 presents the effect of EGCG on the structure of casein micelles. With the uptake of tannins, the micelles became denser with nearly the same average size as indicated by the vertical dashed line. However, the internal structure was completely modified to a random coil polymer-like feature. To gain further insight, we followed the uptake process in a time-resolved manner by rapid mixing of casein micelles and EGCG using a stopped-flow device. The lower inset in Figure 66 shows the evolution of the molecular weight of the globular micelles with a time constant of about 0.18 seconds as EGCG molecules are incorporated. The upper inset of Figure 66 shows the instantaneous modification of the internal structure within the mixing time of about 2 ms. The observed feature corresponds to rapid disintegration of CCP. This suggests that the CCP nanoparticles are localised near the outer region of the micellar core as indicated by the electron density profile in Figure 65b. The full uptake, which corresponds to about a million EGCG molecules per micelle, is reached when EGCG fills the core of the micelles, which then rearranges to the random coil polymer-like structure in a slower process of about 10 seconds.
 |
|
Fig. 66: Modification of the globular and internal structures of casein micelles (2.5 g/L) with the uptake of tannins (EGCG, 2.5 g/L). The lower inset shows the increase of molecular weight of micelles without a significant change in their average size. The upper inset depicts the rapid modification of the internal structure of the micellar core corresponding to the disintegration of the CCP and the development of a random-coil polymer-like organisation. |
In summary, the structure of casein micelles can be modelled using a polydisperse globular core-diffuse shell morphology and an internal structure consisting of a random protein matrix with decorated ellipsoidal calcium phosphate nanoparticles. The diffuse interface of the shell primarily due to ![]() -casein is described by a Gaussian electron density profile. The calcium phosphate nanoparticles are preferentially located in the outer region of the core, this could have made their scattering feature more pronounced despite their low mass content. Our results illustrate that small tannins could be used as a probe to elucidate the internal structure of casein micelles and other natively unfolded proteins. Furthermore, by probing the kinetics by SAXS one may infer nanoscale structural features which are otherwise elusive.
-casein is described by a Gaussian electron density profile. The calcium phosphate nanoparticles are preferentially located in the outer region of the core, this could have made their scattering feature more pronounced despite their low mass content. Our results illustrate that small tannins could be used as a probe to elucidate the internal structure of casein micelles and other natively unfolded proteins. Furthermore, by probing the kinetics by SAXS one may infer nanoscale structural features which are otherwise elusive.
References
[1] D.S. Horne, Curr. Opin. Colloid Interface Sci. 11, 148 (2006).
[2] T. Narayanan, Curr. Opin. Colloid Interface Sci. 14, 409 (2009).
[3] D. Zanchi, T. Narayanan, D. Hagenmuller, A. Baron, S. Guyot, B. Cabane and S. Bouhallab, Europhys. Lett., 82, 58001 (2008).
Principal publication and authors
A. Shukla (a), T. Narayanan (a) and D. Zanchi (b), Soft Matter 5, 2884 (2009).
(a) ESRF
(b) LPTHE, Paris (France)



