- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2006
- Materials Science
- Developing lightweight hydrogen storage materials
Developing lightweight hydrogen storage materials
There is worldwide interest in the use of hydrogen as the basis for a future sustainable energy economy with low carbon emissions. Hydrogen production, storage and utilisation are the three key technological elements of a hydrogen economy and all face significant scientific and technological challenges. One of the key materials challenges is associated with hydrogen storage. Hydrogen contains more chemical energy on a weight for weight basis than any other material but, as the lightest element, it also has a very low energy density.
For hydrogen-fuel cell transportation use – widely regarded as the first major inroad into the hydrogen economy – a suitable material for on-board storage should be able to store a high weight-percent and high volume density of hydrogen and, equally importantly, rapidly discharge and charge this same amount of hydrogen at acceptable temperatures (typically around 50–100°C). This represents a particular challenging set of credentials for an ideal storage material and at present no known material meets these critical requirements.
In 2002, Chen et al. [1] discovered that the system Li3N-Li2NH-LiNH2 could reversibly cycle hydrogen with a theoretical maximum of over 11wt% H2. In practice, only cycling between lithium imide and amide is feasible at realistic pressures and temperatures. Close investigation of by-products of the decomposition of LiNH2, however, showed that ammonia, NH3, is formed as part of the H2 desorption process. This riddle was later resolved bycareful measurements that showed that desorption occurred in two separate steps:
![]()
Although NH3 absorption by LiH is a rapid and efficient process. However, in practice it is difficult to reduce NH3 levels to below 200 ppm, a value that still is high enough to poison current fuel-cell operation.
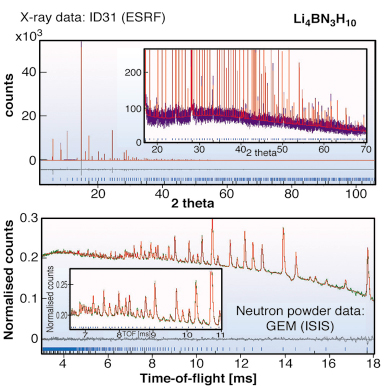
In our search for new materials with similar properties to LiNH2 but with reduced NH3 production, we have investigated various solid solutions of LiNH2 with other materials. Our first study involved a detailed investigation of the LiNH2:LiBH4 phase diagram. Using the ID31 diffractometer under robotic control, we were able to investigate stoichiometries in 1/12 steps across the phase diagram. We discovered a single stable phase with the composition Li4BN3H10. Indexing of the pattern obtained on ID31 indicated a body-centred space group with a 10.6669(7) Å lattice constant and no apparent systematic absences - this is consistent with five possible cubic space groups leading to a significant crystallographic search for the correct structure.
 |
|
Fig. 22: X-ray and neutron powder diffraction patterns of Li4BN3H10. Both datasets are fitted simultaneously to high precision providing strong validation of all aspects of the crystal structure including hydrogen positions. |
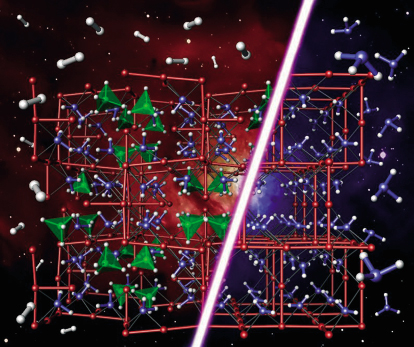
Consideration of the likely density based on the stoichiometry 3LiNH2:LiBH4 led to the conclusion that space groups I23 and I213 were the most likely if the crystal structure is ordered. Chemical considerations then led us to the hypothesis that both NH2 and BH4 groups were situated on an approximate face-centred cubic lattice. In common with LiNH2, the Li atoms were presumed to occupy half of the available tetrahedral sites. Accordingly both I23 and I213 models were tested, with the condition that Li occupancies on all crystallographically independent sites were allowed to vary. I213 was the marginally favoured space group over I23. The difference in goodness of fit is small as NH2 and BH4 are isoelectronic and thus have similar scattering cross sections. Neutron powder diffraction studies were performed on the GEM diffractometer at ISIS. Importantly, the high neutron flux on GEM enabled us to work with natural, unenriched Li4BN3H10. The combination of X-ray and neutron diffraction studies (see Figure 22) led to a precise determination of the full crystal structure, which has been independently corroborated by computational studies [2] as the most precise determination of a number of recent studies [3]. The structure itself is shown in Figure 23 alongside the crystal structure of LiNH2. The similarities between the structure are clearly evident. Remarkably, Li4BN3H10 produces predominantly hydrogen rather than ammonia on desorption with a theoretical ~11wt% H2. Unfortunately having fulfilled the interim of reduced NH3 production, the reversibility of this system is significantly inferior to LiNH2. Optimising all the desirable characteristics of an ideal hydrogen storage material proves to be a difficult task. However the continued study of these systems using ID31 will be a powerful tool in the search for the ideal hydrogen storage material.
 |
|
Fig. 23: Comparison of the crystal structures of Li4BN3H10 and LiNH2 highlighting the fact these two structurally similar materials principally desorb hydrogen and ammonia respectively. |
References
[1] P. Chen, Z. T. Xiong, J. Z. Luo, J.Y. Lin, K.L. Tan, Nature 420, 302-304 (2002).
[2] D. J. Siegel, C. Wolverton, V. Ozolins, Phys. Rev. B 75, 014101 (2007).
[3] Y.E. Filinchuk, K. Yvon, G.P. Meisner, F.E. Pinkerton, M.P. Balogh, Inorg. Chem. 45, 1433-1435, (2006); T. Noritake, M. Aoki, S. Towata, A. Ninomiya, Y. Nakamori, and S. Orimo, Appl. Phys. A: Mater. Sci. Process. 83, 277 (2006).
Principal Publication and Authors
P.A. Chater (a), W.I.F. David (b), S.R. Johnson (c), P.P. Edwards (c), P.A. Anderson (a), Chem. Comm. 23, 2439-2441, (2006).
(a) School of Chemistry, University of Birmingham (UK)
(b) Rutherford Appleton Laboratory (UK)
(c) Inorganic Chemistry Laboratory, Oxford University (UK)



