- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2005
- Soft Condensed Matter
- Dramatic Reduction of Surface Tension of Water
Dramatic Reduction of Surface Tension of Water
Controlling the surface tension of water has been a historical pursuit. Existing surfactants can lower it either as a monomolecular layer on water surface (Langmuir monolayers) or by forming microemulsions. In the former, the bulk water composition is unchanged but the surface tension can be reduced from 72 mN/m to only about 20 mN/m. The microemulsion can make the interfacial tension go to 1 µN/m but changes the water composition. We have shown, through measurement of capillary wave amplitudes using diffuse scattering of X-rays, that a bi-molecular layer of a three-tailed amphiphile, preformed ferric stearate (FeSt), on water surface [1], lowers the surface tension to about 1 mN/m.
Diffuse X-ray scattering experiments, using an 8.04 keV X-ray beam at grazing incidence, i.e. below the critical angle for total external reflection from water, were carried out at beamline ID10B. Preformed FeSt in chloroform solution was spread on water in a Langmuir trough, under He atmosphere, with the diffractometer centre lying on the water surface in the trough. Two different scattering geometries were used, one employing a ‘point’ NaI detector collected the scattered intensity in a vertical plane while the other, using a vertically mounted position-sensitive detector (PSD), collected the vertical scattered intensity profile at different positions on the horizontal plane. Intensity profile along the PSD as well as that collected by the ‘point’ detector is proportional to the out-of-plane structure factor of the film and intensity integrated over the PSD profile is proportional to the surface fluctuation spectrum integrated along propagation direction.
 |
|
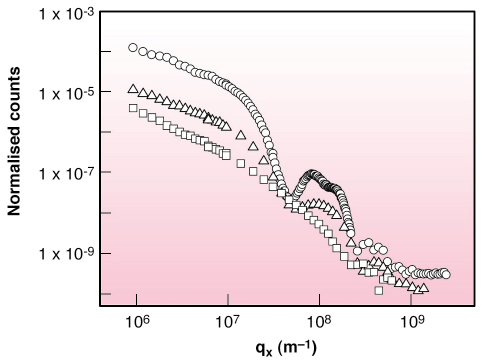
Fig. 56: X-ray scattering in the vertical plane from water (open squares), stearic acid film at |
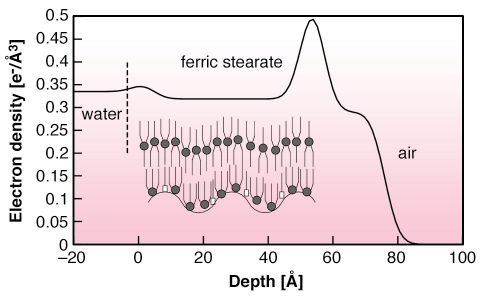
Figure 56 shows the vertically-scattered intensity profiles from water, stearic acid monolayer on ferric chloride solution and film of preformed ferric stearate on water. Whereas the acid monolayer behaves exactly as other Langmuir monolayers [2], the preformed fatty-acid salt film shows a split peak. The average structure factor of FeSt film in the total scattering function yields electron density profile along film depth (Figure 57). It corresponds to the bi-molecular layer shown in schematic, composed of molecules in symmetric configuration, on top of molecules in asymmetric configuration with ferric ions in contact with water. The most interesting feature, shown in Figure 56, as well as in the integrated in-plane scattered profile, is the enhancement by about two orders of magnitude, of the diffuse scattered intensity of the FeSt film at high surface pressure, i.e. high coverage. We ascribe this difference to enhanced conformal capillary wave height fluctuations at air-film and film-water interfaces. The only other possible source of large off-specular scattering is scattering from bimolecular domains, which was estimated from AFM measurements on FeSt films transferred onto Si substrates at low film coverage and was found to be much lower than the observed scattering. We fitted our data using the correlation function for a liquid surface, which gave excellent results over almost four orders of magnitude with surface tension = 1.3 mN/m.
 |
|
Fig. 57: Electron density of compressed ferric stearate film on water, extracted from the X-ray scattering profile shown in Figure 56. Inset: schematics of bi-molecular film with (COO)3Fe (circle) and COOH (square) groups. |
In conclusion, we have demonstrated that a bi-molecular layer of a three-tailed amphiphile drastically enhances capillary wave fluctuations on water surface due to a reduction in surface tension to 1 mN/m. Unlike the usual Langmuir monolayers, this bi-molecular layer does not rupture under compression, but becomes thicker. This behaviour mimics folding of a membrane on a liquid surface and is closely related to the cohesive interaction brought by ferric ions.
This work was carried out under Project 2504-2 of the Indo-French Centre for Promotion of Advanced Research (IFCPAR). We would like to thank IFCPAR for financial assistance.
References
[1] A. Datta, M. K. Sanyal, A. Dhanabalan, and S. S. Major, J. Phys. Chem. B 101, 9780 (1997).
[2] C. Fradin, J. Daillant, A. Braslau, D. Luzet, M. Alba, and M. Goldmann, Eur. Phys. J. B 1, 57 (1998)
Principal Publication and Authors
A. Datta (a), S. Kundu (a), M. K. Sanyal (a), J. Daillant (b), D. Luzet (b), C. Blot (b) and B. Struth (c), Phys. Rev. E, 71, 041604-1 – 041604-7 (2005).
(a) Saha Institute of Nuclear Physics, Kolkata (India)
(b) LIONS, CEA, Saclay (France)
(c) ESRF, Grenoble (France)



