- Home
- News
- Spotlight on Science
- Structure of metallic...
Structure of metallic oxygen
18-08-2009
Solid oxygen undergoes an insulator to metal transition at 96 GPa. Scientists investigated the structural changes that accompany metallisation using single crystal X-ray diffraction.
Share
The search for metallisation in simple molecular systems is a goal of high pressure research motivated by the desire to discover the physical properties of these dense metals and to understand the mechanisms that underlie metallisation. Determination of the structural changes during metallisation is a first step towards this goal. Solid iodine, bromine and oxygen are the only examples of pressure-induced metallisation in simple molecular systems. Recently, the discovery of metallic I2 at 16 GPa was explained by a transient incommensurate modulated structure, nested between the insulating and dissociated metallic states [1]. Oxygen has been shown to metallise at 96 GPa [2], but the structure of metallic oxygen remains unsolved, eluding scientists for more than 20 years.
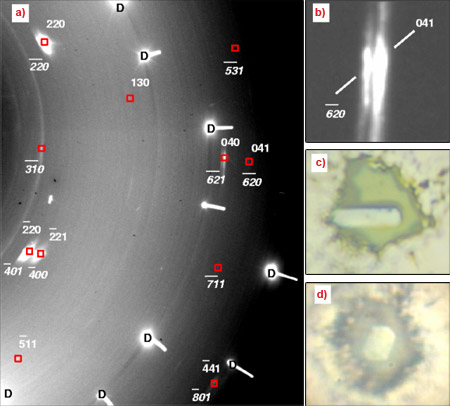
A team of scientists from France and Canada have developed a unique technique based on the growth of high quality single crystals of solid oxygen embedded in helium used as a hydrostatic medium. To reach pressure values beyond one megabar, the use of micro samples was essential. In the present case, the diameter of the high pressure cavity was less than 30 micrometres and the average dimension of the single crystal was less than 10 micrometres. To investigate such small samples requires an experimental setup with very high photon flux and advanced beam focusing capabilities. Angle-dispersive X-ray diffraction experiments were therefore carried out at beamline ID27, and three different oxygen single crystals were studied. In the diffraction patterns obtained, all reflections were found to be well indexed in the monoclinic C2/m unit cell of the low pressure phase up to 96 GPa (ε-O2, stable in between 10 and 96 GPa). During metallisation the diffraction patterns (Figure 1) underwent significant changes characterised by a broadening and a shift of the diffraction spots with increasing pressure. The latter feature indicates the displacive nature of the transition. The structure of the metallic ζ phase presents the same C2/m symmetry as ε-O2 in good agreement with theoretical calculations [3] and a small volume discontinuity could be observed at the transition. A further transformation of metallic O2 into an atomic metal is predicted at much higher pressure, above 250 GPa [3]. However, single crystal X-ray diffraction in that pressure range still represents a formidable technical challenge.
References
[1] T. Kenichi et al., Nature (London) 423, 971 (2003).
[2] S. Desgreniers, Y. Vohra, and A. Ruoff, J. Phys. Chem. 94, 1117 (1990).
[3] Y. Ma, A.R. Oganov, and C.W. Glass, Phys. Rev. B 76, 064101 (2007).
Principal publication and authors
G. Weck (a), P. Loubeyre (a), S. Desgreniers (b), M.Mezouar (c), Single-crystal structural characterization of the metallic phase of oxygen, Phys. Rev. Lett. 102, 255503 (2009).
(a) DIF/DPTA, CEA, Bruyères-le-Châtel (France)
(b) Laboratoire de physique des solides denses, Université d’Ottawa (Canada)
(c) ESRF