- Home
- News
- Spotlight on Science
- A path to new manganites...
A path to new manganites with perovskite structure
15-03-2013
Two new phases of Mn2O3, corundum-type and perovskite-type Mn2O3, were obtained by high-pressure high-temperature synthesis. The observation of the perovskite structure in simple Mn2O3 challenges the main conjecture of perovskite science and technology which so far assumed a strict separation of the A- and B-sites in the crystal structure: the former being occupied by electronically inactive (alkali, alkali-earth, rare-earth) metals and the latter by transition metals.
Share
Perovskites (general formula ABO3) are an extremely common class of oxide materials that find applications in almost all spheres of human activities. They demonstrate enormous chemical and structural flexibility and a large variety of physical properties, e.g. ferroelectricity, superconductivity, colossal magnetoresistance, and multiferroicity. The perovskite structure provides two types of sites for metal cations: octahedrally-coordinated B sites and A sites with variable coordination number. In the overwhelming majority of perovskites, the A and B cations play different roles in the realisation of the material properties: the octahedrally-coordinated transition metal B cations take part in charge transfer and magnetic interactions, whereas the A cations remain electronically inactive. For example, one of the most important and industry-relevant sub-classes of perovskites is manganite perovskites, AMnO3, in which the Mn ions exclusively occupy the B-sites in the crystal structure.
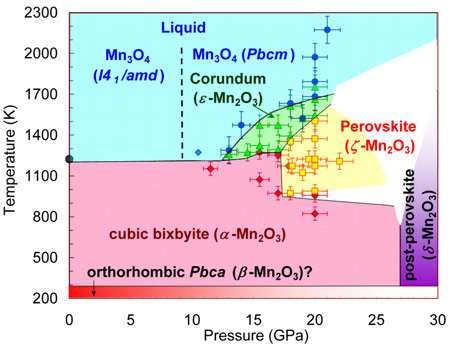
So far there were no cases of binary oxides adopting a perovskite structure. Here, by means of high-pressure and high-temperature synthesis, two new polymorphs of manganese sesquioxide, Mn2O3 recoverable at ambient conditions, namely, corundum-type Mn2O3 and perovskite-type Mn2O3, have been prepared (Figure 1). Previous studies under high-pressure reported two other phases of Mn2O3 - a non-identified monoclinic phase above 20 GPa [1] and a post-perovskite CaIrO3-type phase [2] above 27 GPa. These findings suggest a strong tendency to polymorphism in Mn2O3.
 |
|
Figure 1. Pressure-temperature apparent phase diagram of Mn2O3. |
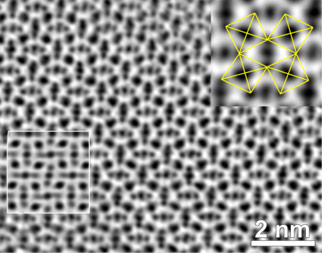
High-resolution transmission electron microscopy studies of perovskite-type Mn2O3 unambiguously showed its perovskite octahedron network (Figure 2). The electron diffraction patterns revealed numerous superstructure reflections suggesting large unit cell parameters. In combined electron diffraction and high-resolution powder diffraction studies carried out at ESRF beamlines ID31 and ID09, we have resolved the structure of the new ζ-Mn2O3 phase in the triclinically-distorted double perovskite type structure (![]() (#2) space group) (Figure 3). The electron energy loss (EEL) spectra of the Mn L23 excitation edge suggest that this phase has the Mn ions in three oxidation states, +2, +3 and +4. With the account of the EELS and bond valence sum data, the valence distribution in the ζ-Mn2O3 may be described as follows: Mn2+(Mn3+)3(Mn3.25+)4O12.
(#2) space group) (Figure 3). The electron energy loss (EEL) spectra of the Mn L23 excitation edge suggest that this phase has the Mn ions in three oxidation states, +2, +3 and +4. With the account of the EELS and bond valence sum data, the valence distribution in the ζ-Mn2O3 may be described as follows: Mn2+(Mn3+)3(Mn3.25+)4O12.
Mn2O3 is the first binary oxide adopting a perovskite-type AA'3B4O12 crystal structure. The proven existence of a ‘binary’ perovskite in a simple oxide advances our understanding of crystal chemistry of geo-materials at conditions relevant to the Earth interior. Furthermore, the case of Mn2O3 shows that high-pressure and high-temperature treatment could be used advantageously to tune the oxidation states of the Mn ions to form new exotic structures, and hence suggesting that new classes of perovskite manganites may be synthesised.
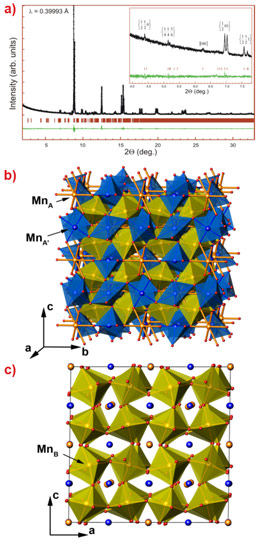 |
Figure 3. Crystal structure of the perovskite-type Mn2O3. |
Principal publication and authors
Perovskite-like Mn2O3: a path to new manganites, S.V. Ovsyannikov (a), A.M Abakumov (b), A.A. Tsirlin (c), W. Schnelle (c), R. Egoavil (b), J. Verbeeck (b), G. Van Tendeloo (b), K.V. Glazyrin (a), M. Hanfland (d) and L. Dubrovinsky (a), Angewandte Chemie International Edition 52, 1494–1498 (2013).
(a) Bayerisches Geoinstitut, Universität Bayreuth (Germany)
(b) EMAT, University of Antwerp (Belgium)
(c) Max Planck Institute for Chemical Physics of Solids, Dresden (Germany)
(d) ESRF
References
[1] T. Yamanaka, T. Nagai, T. Okada, and T. Fukuda, Z. Kristallogr. 220, 938-945 (2005).
[2] J. Santillan, S.-H. Shim, G. Shen, and V. B. Prakapenka, Geophys. Res. Lett. 33, L15307 (2006).
Top image: Crystal structure of the perovskite-type Mn2O3.