- Home
- News
- Spotlight on Science
- Looking inside a...
Looking inside a working chemical reactor with time and space resolved X-ray diffraction
30-08-2012
High-energy X-ray diffraction was used for time and space resolved in operando studies on the zeolite catalyst inside a working catalytic reactor for the methanol-to-olefin process. Scientists were able to correlate growth in size of the unit cell of the zeolite catalyst with its deactivation at different positions in the reactor. The behaviour they observed could be explained by a new kinetic model of the process.
Share
Detailed knowledge of the processes inside an industrial reactor could help chemical manufacturers improve energy efficiency and reduce costs. High-energy X-rays can penetrate to significant depths within a material and therefore have potential for the visualisation of the processes inside a working catalytic reactor. We have used high-energy X-ray diffraction (HEXRD) to investigate the development and location of intermediates in the catalyst bed used for an industrially important reaction. Data were collected in real time from real structural parameters.
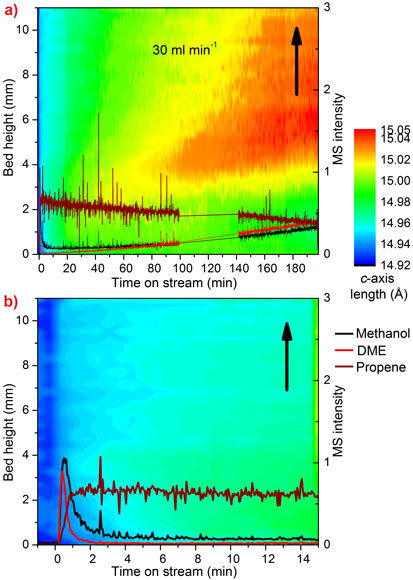
As we move away from oil as a source of petrochemicals, the methanol-to-olefin (MTO) process is becoming a key step in the route from inexpensive, plentiful and even renewable feeds like gas, coal and biomass, to the chemical building blocks we depend on every day. Olefins are a particularly useful group of chemicals which can be transformed into polymers used in anything from plastic bottles to thermal underwear. At present, they are produced mainly by distilling crude oil. The catalyst in the MTO process is the porous zeotype SAPO-34, which suffers from deactivation caused by the build-up of coke in its zeolite cavities. Understanding the progress of this reaction and coke formation in a large, industrial-like reactor is very important in the quest to optimise the process. Our earlier research at the beamline BM01A (Swiss-Norwegian CRG beamline) revealed that the MTO process causes significant expansion of SAPO-34 due to the build-up of hydrocarbon intermediates in the catalyst cavities [1]. The catalyst expands as the content of the cavities changes from relatively small reaction intermediates to large, inactive coke molecules. We collected time- and space-resolved data at the high-energy beamline ID15B and used the expansion of the catalyst as an indication of the progress of the reaction in a 4 mm diameter tube reactor. Diffraction patterns were collected every second as the reactor and heaters were moved down through the beam, giving a series of scans along the vertical axis of the reactor. The output from the reactor was analysed with a mass spectrometer. Each pattern was processed using parametric Rietveld methods (up to 500 datasets at a time) and c-axis expansion was plotted against time and position in the reactor. The resulting contour plots are shown in Figure 1.
Early in the reaction we see that the initial expansion of the catalyst corresponds to an increase in the production of olefins, while the later deactivation of the reactor bed is correlated to the appearance of highly expanded regions of catalyst in the top section of the bed, caused by inactive coke. The point at which the expansion first appears is determined by the flow rate of methanol and the number of catalytic active sites.
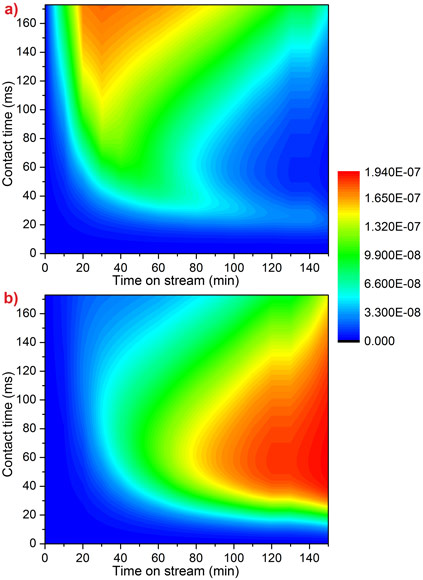
Kinetic modelling reproduces the observed behaviour very well (Figure 2). This model is the first to include the link between methanol feed and increasing coke levels. This knowledge will help to improve the efficiency of the MTO process at an industrial scale.
Principal publication and authors
D.S. Wragg (a), M.G. O’Brien (b), F.L. Bleken (a), M. Di Michiel (c), U. Olsbye (a), H. Fjellvåg (a), Watching the methanol-to-olefin process with time- and space-resolved high-energy operando X-ray diffraction, Angewandte Chemie International Edition 51, 7956–7959 (2012).
(a) inGAP Centre for Research Based Innovation, University of Oslo, Oslo (Norway)
(b) Utrecht University Debye Institute for Nanomaterials Science, Utrecht (Netherlands)
(c) ESRF
References
[1] D.S. Wragg, R.E. Johnsen, M. Balasundaram, P. Norby, H. Fjellvåg, A. Grønvold, T. Fuglerud, J. Hafizovic, Ø.B. Vistad, D. Akporiaye, J. Catal. 268, 290-296 (2009).
Top image: Schematic setup of the method used for time- and space-resolved X-ray data collection at beamline ID15B.