- Home
- News
- Spotlight on Science
- Plutonium dioxide...
Plutonium dioxide nanoparticles puzzle solved through a combination of synchrotron techniques
15-07-2020
A puzzle regarding the fundamental properties of PuO2 nanoparticles was solved by combining information from data collected at beamlines BM20, ID26, and ID15A. This study has relevance for the prediction of the fate of environmental plutonium from contaminated soils and waste storage.
Nanoscience sometimes produces results that are more mystifying than in any other discipline. Changes in the plutonium dioxide (PuO2) particle size from bulk to nano could have a dramatic effect on PuO2 properties. Indeed, plutonium is one of the most complex and fascinating chemical elements in the periodic table. In environmentally-relevant conditions, Pu may exist and even co-exist in four oxidation states, from Pu(III) to Pu(VI). It has been discovered that Pu may be transported by groundwater from contaminated sites on a scale of kilometres when bound to mineral or organic colloids. Recently, it was repeatedly found that PuO2+x nanoparticles (NPs) are formed during interfacial processes between Pu in different initial oxidation states and various mineral surfaces (hematite, goethite, quartz, and mica) and bacteria. All these results indicate the high importance of PuO2 NPs in the context of the environmental behaviour of Pu. To improve our understanding of Pu properties, it is necessary to use licensed laboratory facilities to handle radioactive material safely, to combine this with the most powerful experimental methods and to complement the results with theoretical studies. X-rays emitted by a synchrotron can be used to study the atomic structure of such materials because of their penetrative behaviour and their sensitivity to the local environment and the electronic structure of the selected element.
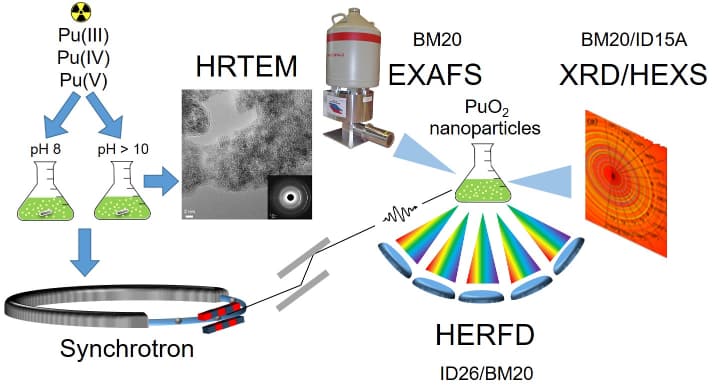
A systematic investigation of PuO2 NPs was carried out, synthesised using environmentally and waste storage relevant conditions by varying the pH (e.g. pH 8 and pH>10) and the precursor (Pu(III), Pu(IV), Pu(V)). The synthesised PuO2 NPs were characterised by a variety of experimental methods: extended X-ray absorption fine structure (EXAFS) at BM20, X-ray absorption near-edge structure (XANES) in high energy resolution fluorescence detection (HERFD) mode at the Pu L3 edge at BM20 and at the Pu M4 edge at ID26, high energy X-ray scattering (HEXS) at ID15A and X-ray diffraction (XRD) at BM20. The theoretical interpretation of the experimental data was based on the Anderson impurity model (AIM) and ab initio calculations. Figure 1 shows a schematic illustration of the research methodology applied to PuO2 NPs studies.
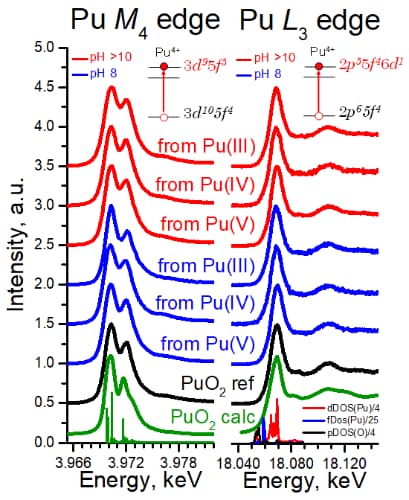
The most intriguing question concerning PuO2 nanoparticles is the potential presence of various oxidation states of plutonium (referred to as PuO2+x). The formation of PuO2 NPs has already been investigated by various methods, but none has provided an unambiguous answer regarding the Pu oxidation state. In this work, HERFD was applied at beamlines ID26 and BM20 to obtain straightforward information about the Pu oxidation state for the first time (Figure 2). The HERFD data at the Pu L3 edge confirm the presence of the Pu(IV) oxidation state as the dominant valence of PuO2 NPs, as seen from the positions of the Pu white line. However, a minor white line energy shift among the samples is observed (within 0.2-0.4 eV), which prevents exclusion of tiny amounts of other oxidation states being present. Previous experiments showed that HERFD measurements at the actinide M4 edge, characterised by the actinide 3d-5f electronic transitions, are very powerful for oxidation state identification [1]. The Pu M4 HERFD spectrum of PuO2 shows a sharp peak at ∼3970 eV due to the transitions from the 3d3/2 core level to the unoccupied 5f5/2 level and a shoulder at higher energy. This splitting of the Pu M4 main edge transitions is reproduced very well by theoretical calculations and relates to the multiplet splitting of the Pu 5f states. These results confirm the presence of only the Pu(IV) oxidation state in all PuO2 NPs and settle the controversial debates of the last years about the presence of other oxidation states in nanosystems.
In general, spectroscopic methods with standard resolution have been used extensively in studies of actinide systems in the past years. One of the most popular methods is EXAFS at the Pu L3 edge, which has been carried out multiple times on PuO2 NPs by different research groups, but results are heavily debated and are not in agreement with one another. The main discrepancy is related to the different interpretations of the first coordination sphere, which refers to Pu-O interaction and can be classified as:
- a single Pu-O interaction similar to the one in PuO2;
- several Pu-O interactions (with different coordination of O atoms);
- several Pu-O interactions, including Pu in various oxidation states.
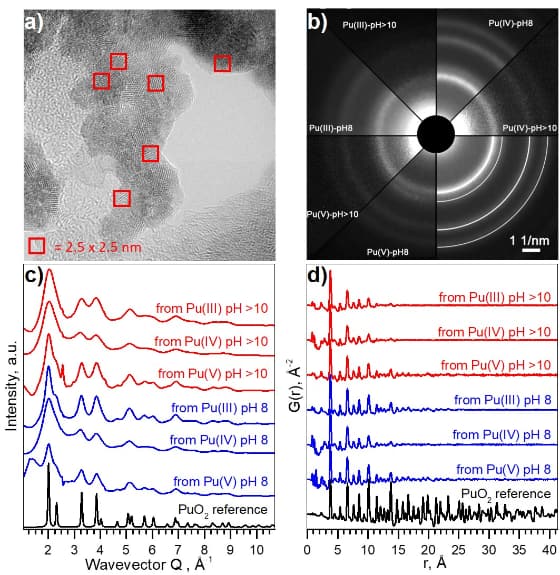
A combination of techniques and theoretical calculations was used in order to find the most consistent model. Pu-O interactions with Pu in various oxidation states can be discarded using the HERFD results. Several complementary methods were used to verify the presence or absence of different Pu(IV)-O contributions. The agreement of the results from the EXAFS shell fit, Monte-Carlo EXAFS simulations, EXAFS Landweber iteration approach and from HEXS indicates unequivocally that only one Pu(IV)-O interaction with a rather symmetric distance distribution (similar to the PuO2 reference) is present for all six PuO2 NPs samples.
Moreover, XRD and HEXS experiments, performed at the ID15A beamline (Figure 3) show that PuO2 NPs are unexpectedly homogeneous and monodisperse. The crystalline structure of the NPs is similar to that of bulk PuO2. It is surprising and not yet understood why NPs of 2.5 nm are always formed. There are certain difficulties in obtaining bigger PuO2 NPs (size 5-10 or 15 nm) via the chemical precipitation method.
All the collected information plays an important role in explaining plutonium chemistry under real conditions. It will help to advance, to model and to predict long-term Pu release from deep underground repositories of nuclear waste and contaminated sites [2].
Principal publication and authors
E. Gerber (a,b,c), A. Yu. Romanchuk (c), I. Pidchenko (a,b), L. Amidani (a,b), A. Rossberg (a,b), Ch. Hennig (a,b), G.B.M. Vaughan(d), A. Trigub (e), T. Egorova (c), S. Bauters (a,b), T. Plakhova (c), M. O.J.Y. Hunault (f), S. Weiss (b), S.M. Butorin (g), A.C. Scheinost (a,b), S.N. Kalmykov (c,e) and K.O. Kvashnina (a,b,c), The missing pieces of the PuO2 nanoparticles puzzle, Nanoscale (2020); doi: 10.1039/d0nr03767b.
(a) The Rossendorf Beamline at ESRF (BM20), The European Synchrotron, Grenoble (France)
(b) Helmholtz-Zentrum Dresden-Rossendorf (HZDR), Institute of Resource Ecology, Dresden (Germany)
(c) Lomonosov Moscow State University, Department of Chemistry, Moscow (Russia)
(d) ESRF
(e) National Research Centre “Kurchatov Institute”, Moscow (Russia)
(f) Synchrotron SOLEIL, L’Orme des Merisiers (France)
(g) Molecular and Condensed Matter Physics, Department of Physics and Astronomy, Uppsala University, Uppsala (Sweden)
References
[1] K.O. Kvashnina, A.Y. Romanchuk, I. Pidchenko, L. Amidani, E. Gerber, A. Trigub, A. Rossberg, S. Weiss, K. Popa, O. Walter, R. Caciuffo, A.C. Scheinost, S.M. Butorin, and S.N. Kalmykov, Angew. Chemie Int. Ed. 58, 17558 (2019).
[2] A.Y. Romanchuk, T. V. Plakhova, A.V. Egorov, T.B. Egorova, P.V. Dorovatovskii, Y.V. Zubavichus, A.A. Shiryaev, and S.N. Kalmykov, Dalt. Trans. 47, 11239 (2018).