- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2016
- Electronic structure, magnetism and dynamics
- Nanostructured palladium-ceria catalyst for anion exchange membrane fuel cells
Nanostructured palladium-ceria catalyst for anion exchange membrane fuel cells
One of the biggest obstacles to the diffusion of fuel cells is their cost, in large part due to platinum catalysts. Here, a platinum-free fuel cell was studied that employs a mixed carbon-CeO2 supported palladium anode catalyst and produces peak power densities of more than 500 mW cm-2.
Recent analyses have shown that among fuel cell stack components around 45% of the cost comes from the platinum catalyst. Therefore, a complete removal of platinum from fuel cells and replacement with metals that are less expensive and more abundant in nature is crucial. As an alternative to traditional fuel cells that operate under corrosive acidic conditions with membranes like nafionTM, the use of an alkaline membrane fuel cell (AEM-FC) may reduce costs by avoiding the use of platinum. The main obstacle to the development of this type of fuel cell is the challenge of overcoming poor hydrogen oxidation (anode) kinetics in alkaline media.
We present a nanoparticle palladium based catalyst (10 wt% Pd) with a composite support made of Vulcan XC-72 carbon and CeO2 (C-CeO2) which exhibits enhanced kinetics in alkaline media. Our fuel cell containing this catalyst performs with no platinum in the catalysts (Pd at the anode and Ag at the cathode) and produces 500 mW cm-2 peak power output running on dry hydrogen and air.
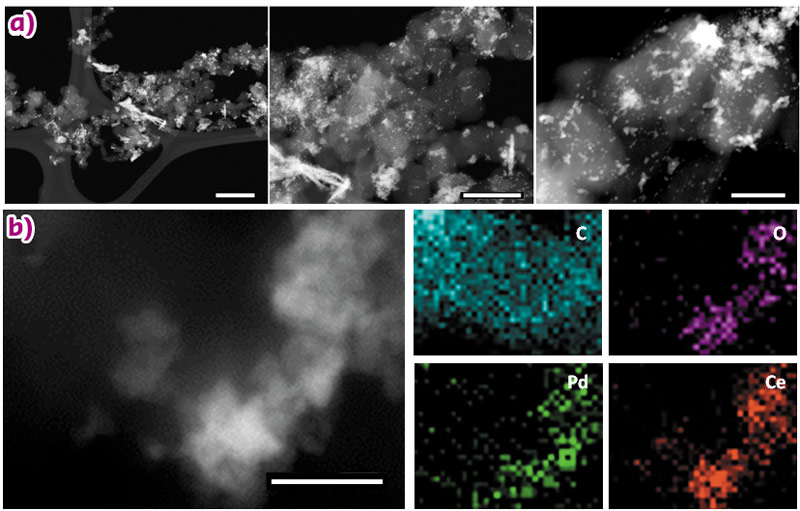
High angle annular dark field (HAADF) scanning transmission electron microscopy (STEM) and high-resolution transmission electron microscopy (HR-TEM) were used to study the catalyst morphology. Z-contrast STEM micrographs of Pd/C-CeO2 are shown in Figure 25a. The CeO2 structures appear brighter than carbon on the STEM images. Palladium nanoparticles are visible only on the carbon part of the sample due to poor resolution between Pd and CeO2 (mean size of carbon supported nanoparticles 2.0 nm). Therefore, we used STEM-EDX (energy dispersive X-ray) elemental map analysis to investigate the Pd distribution over both the carbon and ceria parts of the catalyst. A representative area of the catalyst is shown in Figure 25b, that shows a notable accumulation of Pd on the ceria-rich regions. Our electrochemical investigations show that the Pd-CeO2 interaction is responsible for the enhanced activity of this catalyst.
 |
|
Fig. 25: a) STEM micrographs of Pd/C-CeO2 (scale bars from left to right; 200 nm, 100 nm and 50 nm respectively) and b) STEM image of Pd/C-CeO2 and related EDX maps for C, O, Pd and Ce respectively (scale bar is 20 nm). |
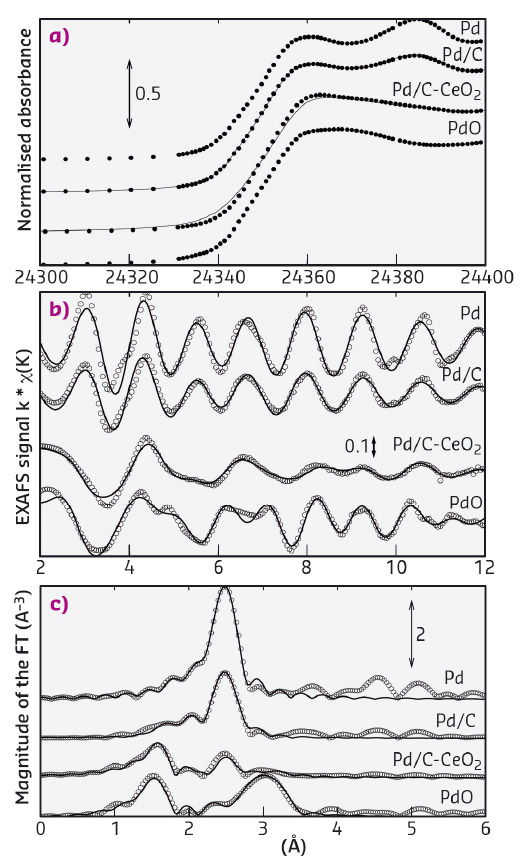
Strong Pd-CeO2 interactions would have a significant effect on the oxidation state of the Pd due to the oxide spillover capacity of ceria that would leave most of the Pd in the oxidised form. To verify this, the Pd oxidation state in the Pd/C-CeO2 catalyst was investigated by X-ray absorption spectroscopy (XAS) and compared to a simple Pd on carbon catalyst (Pd/C) with the same loading. XAS data were obtained at beamline BM08 (LISA CRG). Spectra were also collected on PdO and on a foil of metallic Pd as standards. In Figure 26a, a comparison of the XANES (X-ray absorption near-edge structure) spectra clearly shows that Pd in Pd/C-CeO2 is mostly oxidised, while in Pd/C the palladium is prevalently in its metallic state. EXAFS (extended X-ray absorption fine structure) analysis was carried out modelling the data with two components, i.e. metallic Pd and PdO. The raw EXAFS data and related Fourier transforms are shown in Figures 26b and 26c respectively. The EXAFS analysis shows PdO accounts for 87 wt% of the total Pd content in Pd/C-CeO2. This is unusual as carbon supported Pd nanoparticles (ca. 2 nm in diameter) are usually found to be at least 50% metallic. As expected, only 17 wt% of PdO was found in the Pd/C system. The XAS data therefore shows that the Pd exists primarily as oxide for the C-CeO2 supported catalyst also confirming that Pd is largely supported on the ceria regions.
 |
|
Fig. 26: a) XAS at the Pd Kα edge of Pd/C-CeO2 and Pd/C (XANES spectra of Pd-foil and PdO standards are also shown), b) EXAFS data and the related c) Fourier transforms at the Pd Kα edge. |
In summary, we studied an entirely Pt-free fuel cell that produces a peak power density of over 500 mW cm-2. This performance is due to a Pd/C-CeO2 catalyst where the Pd-CeO2 interactions enhance hydrogen oxidation kinetics.
Principal publication and authors
A Pd/C-CeO2 anode catalyst for high-performance platinum-free anion exchange membrane fuel cells, H.A. Miller (a), A. Lavacchi (a), F. Vizza (a), M. Marelli (b), F. Di Benedetto (c), F. D’Acapito (d), Y. Paska (e), M. Page (e) and D.R. Dekel (f), Angew Chem Int Edit 55, 6004-6007 (2016); doi: 10.1002/anie.201600647.
(a) CNR-ICCOM, Firenze (Italy)
(b) CNR-ISTM, Milan (Italy)
(c) Department of Earth Sciences, Università di Firenze (Italy)
(d) CNR-IOM-OGG c/o ESRF, Grenoble (France)
(e) CellEra, Caesarea (Israel)
(f) Technion – Israel Institute of Technology, Haifa (Israel)



