- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2015
- Structural biology
- The fifth copper binding site of the prion protein acts as a molecular switch for prion conversion
The fifth copper binding site of the prion protein acts as a molecular switch for prion conversion
The conversion of the prion protein (PrPC) into a misfolded isoform in the brain causes prion diseases, a group of transmissible neurodegenerative disorders affecting humans and animals. The structural events underlying this conversion have escaped detailed characterisation due to the insoluble nature of prions. A novel copper-mediated mechanism has been identified that acts as a “switch” that turns PrPC into its pathological alter ego.
In its physiological form, the prion protein (PrPC) is beneficial and involved in several neuronal processes such as neuronal growth and differentiation and brain metal homeostasis. The latter function is attributed to the prion protein’s ability to bind copper suggesting that PrPC possesses metalloprotein function.
A conformational change of PrPC into a “bad” form is responsible for a class of neurodegenerative diseases called prion diseases that include “mad cow disease” or Creutzfeldt-Jakob disease in humans. This form is termed “prion” or PrPSc and, once formed, acts as a template for the conversion of additional PrPC to PrPSc facilitating a build-up of misfolded protein and subsequent neurodegeneration.
Although copper binding seems to be involved in many physiological PrPC processes, the interplay between copper and prion conversion poses intriguing issues. There are five potential copper binding sites clustered along the unstructured N-terminal domain: four within the octapeptide repeats (OR) region (residues 60-91) and one close to the globular domain called non-OR or “fifth” copper binding site (residue 92-111) [1]. Interestingly, copper coordination to the non-OR region has garnered interest due to the possibility that this interaction may impact prion conversion.
Here, by means of extended X-ray absorption fine structure (EXAFS) spectroscopy, cell-biology approaches and molecular dynamic simulations, we have investigated the influence of copper coordination in the non-OR region on prion conversion.
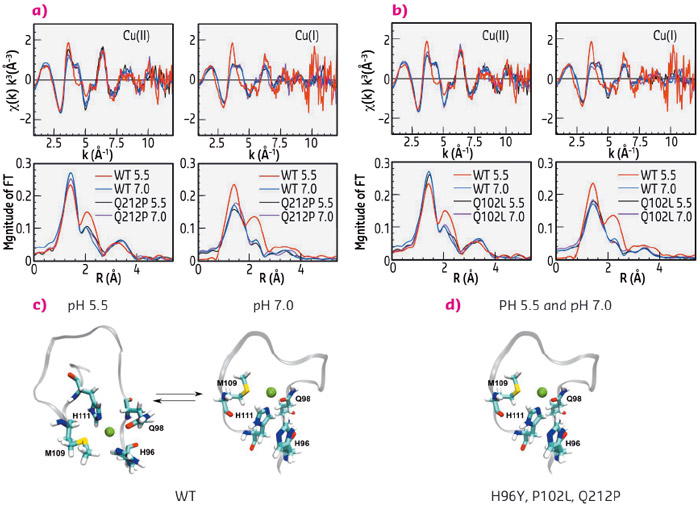
Using X-ray absorption spectra collected at beamline BM30B FAME, we determined the copper coordination in the wild type (WT) human prion protein (HuPrP) and in pathological mutants at both pH 7.0 and pH 5.5. We found that mutations (H96Y, P102L and Q212P) and pH changes cause a dramatic modification on both Cu(II) and Cu(I) coordination in the non-OR region. In the WT HuPrP Cu(II) and Cu(I) are anchored to His96 and His111 only at pH 5.5, while at pH 7 copper in both oxidative states is coordinated only by His111. Conversely, in the mutants copper is bound only to H111 independently of the pH (Figure 122).
 |
|
Fig. 122: Copper coordination in the WT and mutant human prion protein. k3-weighted EXAFS spectra and Fourier transforms of Cu(II) and Cu(I) bound to WT HuPrP(90–231) and Q212P at pH 5.5 and 7.0 (a) and of Cu(II) and Cu(I) bound to WT HuPrP(90–231) and P102L at pH 5.5 and 7.0 (b). Schematic representations of copper binding sites in the WT HuPrP(90–231) (c) and in the mutants (d) at both pH 5.5 and 7.0. |
The observed structural differences in the copper coordination among WT and pathological mutants at pH 5.5 may have relevant physiological implications since this alteration in the copper binding site might trigger PrPC to PrPSc conversion. Hence, the non-OR region could be an important “hot spot” for prion conversion. To understand the implications of our EXAFS data, we performed cell-based assays. We used neuronal cell models expressing PrPC in which the N-terminal His residues within the OR and non-OR regions were substituted by tyrosine (Tyr) and we evaluated the effect of single His to Tyr substitutions in the context of prion conversion. Only the H96Y mutation promoted significant prion conversion and generation (Figure 123a, b). His to Tyr96 substitution removes one crucial copper ligand, thus it may allow the effect caused by altered copper coordination to be linked with prion conversion.
 |
|
Fig. 123: The non-OR H96Y mutation promotes prion conversion in ScN2a cells (a) and accelerates prion protein fibrillisation (b). PrPC coordinating copper with one His residue in the non-OR region is more prone to the conversion at acidic pH condition. We propose a model where His96 and His111 represent the N-terminal switch for prion conversion in the PrPC (c). |
These findings suggest a role for the non-OR region as a critical molecular switch for prion conversion. We therefore argue that copper bound to the non-OR region may stabilise this segment when coordinated by His96 and His111. Our study highlights the importance of the non-OR region for prion conversion and suggests a model in which PrPC coordinating copper using only one His may be more prone to the conversion in acidic conditions (Figure 123c).
Principal publication and authors
The non-octarepeat copper binding site of the prion protein is a key regulator of prion conversion, G. Giachin (a), P.T. Mai (b), T.H. Tran (b), G. Salzano (b), F. Benetti (b), V. Migliorati (c), A. Arcovito (d), S.D. Longa (e), G. Mancini (f), P. D’Angelo (c) and G. Legname (b), Sci Rep. 5, 15253 (2015); doi: 10.1038/srep15253.
(a) ESRF
(b) Department of Neuroscience, Scuola Internazionale Superiore di Studi Avanzati (SISSA), Trieste (Italy)
(c) Department of Chemistry, Sapienza University of Rome (Italy)
(d) Istituto di Biochimica e Biochimica Clinica, Università Cattolica del Sacro Cuore, Rome (Italy)
(e) Department of Medicine, Public Health, Life and Environmental Science, University of L’Aquila, Coppito Aquila (Italy)
(f) Scuola Normale Superiore, Pisa (Italy)
References
[1] P. D’Angelo et al., Biochemistry 51, 6068–6079 (2012).



